Royal Institution Christmas Lectures 2012
I’ve been watched the Royal Institution Christmas lectures with my family, and ever they are brilliantly presented exploration of an area of science. This year it is the turn of Peter Wothers “The Modern Alchemist” to explain about the chemicals in the air we breath and the water we drink. Hopefully inspiring the next generation of scientists and hopefully convincing people that “chemicals” far from being man-made toxins are in fact essential for all human life.
If you have ever wanted to know what the reaction of caesium with fluorine is like then have a look at lecture two.
The lectures are televised on BBC 4 and are available on iPlayer
Further behind the scenes clips and more information is available on the Royal Institution website
The two most reactive elements in the Periodic Table. In preparation for the 2012 Christmas Lectures Dr Peter Wothers heads off to the University of Leicester to conduct an extraordinary experiment - reacting the most reactive metal in the periodic table (Caesium) with the most reactive non-metal (Fluorine). Due to the extreme reactivity of the two elements, Fluorine expert Professor Eric Hope is on hand to enable the experiment to be conducted safely in a unique set of apparatus. We believe this is the first time the reaction has been caught on camera.
Seasons Greetings
Wishing Everyone a successful New Year.
As in previous years money saved on cards will be donated to MS research.
RSC aquires The Merck Index
The Merck Index, is to join the highly acclaimed publishing portfolio of the Royal Society of Chemistry.
The RSC already plans significant development of The Merck Index online, and will continue to develop and update.
I wonder if this will be integrated into ChemSpider? When you also consider that the RSC is taking over the Chemical Database Service it is clear that the RSC is moving to increase it’s support of online chemistry.
Following a tendering exercise earlier this year the EPSRC will be renewing the Chemical Database Facility, with the Royal Society of Chemistry being the preferred bidder. Contract negotiations are being pursued and should be in place by the end of this year. Details of the renewed service will be made known in due course and the RSC will be working with Daresbury to ensure a seamless transfer to the new system.
SMARTCyp Updated
SMARTCyp 2.3 has been released with some additional improvements including: Improved energies for N-oxidations Empirical correction for unlikely N-oxidations of tertiary alkylamines A filtering functionality for excluding compounds with very low activation barriers to CYP-mediated oxidations A smiles string can now be input directly on the command line using the -smiles flag. Available as usual at http://www.farma.ku.dk/smartcyp The science behind the improved N-oxidations and the empirical correction has also been published in a paper in Angewandte Chemie: DOI
Modelling the Drug Discovery Pipeline
An interesting publication in the latest issue of Journal of Cheminformatics in which Melvin Yu uses Monte Carlo simulations to look at the Drug Discovery pipeline DOI. With any analysis of this kind it is easy to argue that it is too simplistic however it does raise some useful discussion points. In particular, the idea that simply attempting to improve drug discovery productivity by simply increasing the size of existing working groups may not necessarily be the best solution.
This also sounds familiar
Simulations also predict that the frequency of compounds to successfully pass the candidate selection milestone as a function of time will be irregular, with projects entering preclinical development in clusters marked by periods of low apparent productivity
Perhaps a greater number of independent smaller research units is a more attractive model?
FiercePharma
FiercePharma are obviously on a recruitment drive, I have to confess I’m already a subscriber. Makes an interesting read over the early morning cup of coffee.
Updated Frament Screening Pages
I’ve updated the fragment-based screening pages, added new screening technologies, added a couple of new vendors and updated the fragment collection profiles based on the current fragment collections that vendors have provided.
Zinc databases Updated
I see the Zinc databases have been updated (ZINC, is a free database of commercially-available compounds for virtual screening)
Lead-like, 5.7M, today Shards, 55K, Oct 15 Frag-like, 636K, Oct 15 Leads-now, 2M, Oct 15 Frags-now, 440K, Oct 12
There are more databases here
Bioisosteres Pages updated
I’ve added a few more examples to the Bioisosteres pages. Many thanks to the readers who sent the suggestions.
A review of Bioisosteres in Medicinal Chemistry
When I look at the weblogs of the Drug Discovery Resources section of my website it is clear that two sections are of particular interest, Fragment-Based screening attracts a steady stream of readers and the other area that is very popular is the section on Bioisosteres. The former because it is an increasingly popular, effective and low cost entry point into screening for leads, the latter presumably because it provides a great way to generate ideas of what one might do next.
I’m not aware of a book dedicated solely to Bioisosteres so I was delighted to hear about a new book in the area Bioisosteres in Medicinal Chemistry edited by Nathan Brown.
Having had time to read through the book I thought I’d post my thoughts.
A bioisostere is a molecule resulting from the exchange of an atom or of a group of atoms with an alternative, broadly similar, atom or group of atoms. The objective of a bioisosteric replacement is to create a new molecule with similar biological properties to the parent compound, but perhaps with improved physicochemical or ADME properties. Whilst the experienced Medicinal Chemist will have a toolbox of such replacements, this book is an excellent resource that provides a logical framework to this area of drug discovery that will be useful to all.
The book is organised into four section, the first deals with classical replacements, the second the discovery of bioisosteres based on database mining, the third section deals with physicochemical properties and shape, the final section includes the application of bioisosterism in a drug discovery project.
The book starts by giving a short history of bioisosteres and several examples of the attempts to define what is exactly meant by a bioisostere, it then quickly moves onto description of the development of many of the classical bioisosteres, giving plenty of examples and references. The influence of the bioisostere on ADME properties is also introduced again with plenty of key examples. The second section starts by describing the evolution of the Bioster database that has been created by abstracting examples from the Medicinal Chemistry literature. There is also a description of how it might be used as a source of bioisosteric replacements. In contrast mining the Cambridge Structural Database has the potential to identify unexpected replacements based on the arrangement of potential non-bonding interactions. As mentioned agove one of the key drivers for looking at bioisosteres is to improve ADME properties, and mining a large database of metabolic stability data has allowed the identification of transformations that are more likely to improve stability. The third section deals with physicochemical properties, molecular topology and shape, describing in some detail the in silico techniques that can be used to scaffold hop to identify novel frameworks. The final section describes the Drug Guru project, and the use of the molecular field based screening in a NPY-Y5 project.
In summary the book is an excellent starting point for new medicinal chemists and a useful resource for more experienced scientists to dip into to generate ideas. in summary every chemist involved in drug discovery would benefit from access to this book.
Viewing docking results in Vortex
This may be of interest.
I recently wrote a review of ForgeV10 from Cresset in which I actually imported the results into Vortex to do the analysis. There were however two issues with doing this, firstly interpretation of the 3D structures is sometimes difficult, this can be resolved by creating a 2D rendering of the structure. The other issue is trying to interpret the docking pose whilst looking at the analysis of the results in say a Vortex scatter plot.
I’ve been working with Mike Hartshorn and the people at Dotmatics who have incorporated OpenAstexViewer (a 3D molecule viewer) into the application you can read the full article here..
A virus that kills cancer
Ad5[CgA-E1A-miR122]PTD is a virus created by Professor Magnus Essand that has been shown in animal models to infect and kill cancer cells. The work has been published and is thus not patentable, however they hope to develop the virus with the support of charitable funding and crowdsourcing.
If you would like to find out more or donate visit http://www.fundingjar.com/projects/130/project-info/
Bioisosteres in Medicinal Chemistry
I mentioned this book in the past and I have just been told it is now available on Amazon.
Written with the practicing medicinal chemist in mind, this is the first modern handbook to systematically address the topic of bioisosterism. As such, it provides a ready reference on the principles and methods of bioisosteric replacement as a key tool in preclinical drug development. The first part provides an overview of bioisosterism, classical bioisosteres and typical molecular interactions that need to be considered, while the second part describes a number of molecular databases as sources of bioisosteric identification and rationalization. The third part covers the four key methodologies for bioisostere identification and replacement: physicochemical properties, topology, shape, and overlays of protein–ligand crystal structures. In the final part, several real–world examples of bioisosterism in drug discovery projects are discussed. With its detailed descriptions of databases, methods and real–life case studies, this is tailor–made for busy industrial researchers with little time for reading, while remaining easily accessible to novice drug developers due to its systematic structure and introductory section.
Updated Pages
I’ve updated the page of online databases to include databases that contain biological information that might be useful.
I’ve also added a link to a series of presentations and posters available from Sirius on the physicochemical properties page.
Fragment methods in drug discovery: the potential grows
An interesting set of articles focussed on Fragment methods published in Drug Discovery Today.
Fragment methods have now become well established within the repertoire of drug discovery technologies used within the pharma and biotech industries. Success has been repeatedly demonstrated in the application of fragment methods as the basis for the discovery of drug candidates with attractive physicochemical properties for soluble protein targets….In this issue of Drug Discovery Today, Editor’s Choice highlights four recent papers that consider how to make the best use of fragments both in terms of their optimisation and their application more broadly in drug discovery. The papers featured are all available as free downloads, so please have a look at them; I’m sure that you will find them interesting and thought provoking.
Fragment Collections
I’ve updated the page describing the different fragment collections.
17th RSC/SCI Medicinal Chemistry Symposium
The first circular for the 17th RSC/SCI Medicinal Chemistry Symposium is available for download. The meeting will be held at Churchill College Cambridge UK, 8-11 September 2013. Always a hugely popular meeting so worth registering your interest early.
Drug Discovery Stage Definitions
Whilst the Drug Discovery process is continuous and can vary depending on target it is often useful to split the process into stages with key milestones and targets clearly defined and met before a project moves from one stage to the next.
ODDT Publication
Investigators of rare and neglected diseases can access some of the latest research in the field, plus data about the disorders themselves, through a recently launched free app for Apple devices.
Bioisosteres Updated
Fragment Screening Publication
http://dx.doi.org/10.1177/1087057112445785
Fragment Profiles Updated
Bioisosteres in Medicinal Chemistry by Nathan Brown
Big Pharma in Crisis
http://www.samedanltd.com/magazine/11/issue/175/article/3271
CYP450 Induction
Magic methyl
Methyl Effects on Protein–Ligand Binding
Cheryl S. Leung, Siegfried S. F. Leung, Julian Tirado-Rives, and William L. Jorgensen
A literature analysis of >2000 cases reveals that an activity boost of a factor of 10 or more is found with an 8% frequency, and a 100-fold boost is a 1 in 200 event….The greatest improvements in activity arise from coupling the conformational gain with the burial of the methyl group in a hydrophobic region of the protein.
DOI: 10.1021/jm3003697
ODDT Released
The idea behind ODDT is that there are many rare of neglected diseases that might benefit from collaborative efforts from scientists from multiple disciplines, ODDT is an application that supports informal interactions, provides a means to explore relevant information in a flipboard like interface in particular information tagged by other scientists with similar interests. The image below gives you an idea of the topics currently discussed.
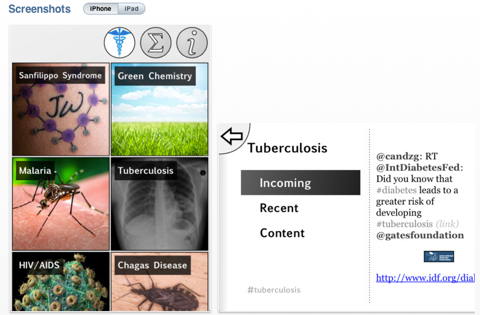
This slideshow explains the genesis of the project, and how it has evolved.
So why not fire up iTunes and download the free app and get involved?
Fragment Screening Pages Updated
I’ve just updated the pages devoted to fragment-based screening
Fragment Collections
Fragment Collection Profiles
Fragment-Based Screening
Known Fragment Hits
Many thanks to all who provided details of their fragment collections.
Bioisosteres updated
ChEMBL 13
ChEMBL 13 has been released.
This release includes updates to the manually extracted Medicinal Chemistry literature, updates to OrangeBook drug approvals and a update from PubChem BioAssay. This release also contains data sets related to screening against human African Trypanosomiasis and Chagas disease. Both data sets have been deposited by the Drugs for Neglected Diseases Initiative (DNDi).
This latest version of the ChEMBL database contains:
- 1,304,115 compound records
- 1,143,682 distinct compounds
- 617,681 assays
- 6,933,068 bioactivities
- 8,845 targets
- 44,68 documents
- 8 data sources
The data can be downloaded from the ChEMBL website.
Added links to MACiE
I’ve added links to the MACiE database (Mechanism, Annotation and Classification in Enzymes), to provide more information on the mechanisms of the Aspartic, Serine and Cysteine Proteases.
Also started a page on metalloproteases.
Biology and Pathology of the Malaria Parasite, 14 - 16 May 2012
Registration and abstract deadlines will be closing on 1st March 2012
Pleae note that registration without abstract submission is also possible.
Conference website: www.embl.de/training/events/2012/BMP12-01
Venue: EMBL Advanced Training Centre, Heidelberg, Germany
This annual conference will bring together malaria researchers from Europe and overseas in order to present and share recent groundbreaking findings on fundamental malaria research.
Topics covered include: - Immunobiology and Pathophysiology - Parasite Molecular and Cell Biology - Vector Parasite Interactions - Modelling and Systems Biology We also invite you to register for a two and a half day workshop designed to provide you with training in effective use of several online resources relevant for Plasmodium research, including: - GeneDB (http://GeneDB.org) - PlasmoDB (http://PlasmoDB.org) - VectorBase (http://VectorBase.org) The workshop will start at 9am on Saturday May 12th and run until Monday May 14th. You are welcome to circulate this announcement to interested members and groups within your institute.
We look forward to welcoming you in Heidelberg, Germany.
Matthew Berriman, Wellcome Trust Sanger Institute, United Kingdom Peter Bull, KEMRI Wellcome Trust, United Kingdom Flaminia Catteruccia, University of Perugia, Italy Lisa Ranford-Cartwright, University of Glasgow, United Kingdom Till Voss, Swiss Tropical and Public Health Institute / University of Basel, Switzerland
If you have any questions, please do not hesitate to contact Tim Nuernberger. Email: tim.nuernberger@embl.de European Molecular Biology Laboratory
More bioisosteres added
I’ve updated the bioisosteres section, adding a number of new examples of bioisosteric replacements.
More Ester and Amide bioisostere examples
I’ve updated Bioisosteres section adding several new examples to the Ester and Amide bioisosteres.
Website Update
I’ve now finished updating the website. I’ve changed the host, redesigned the theme and updated almost every page.
Feel free to have a look around and let me know of any issues or suggestios.
