Bioisosteric Replacements
Acid Bioisosteres
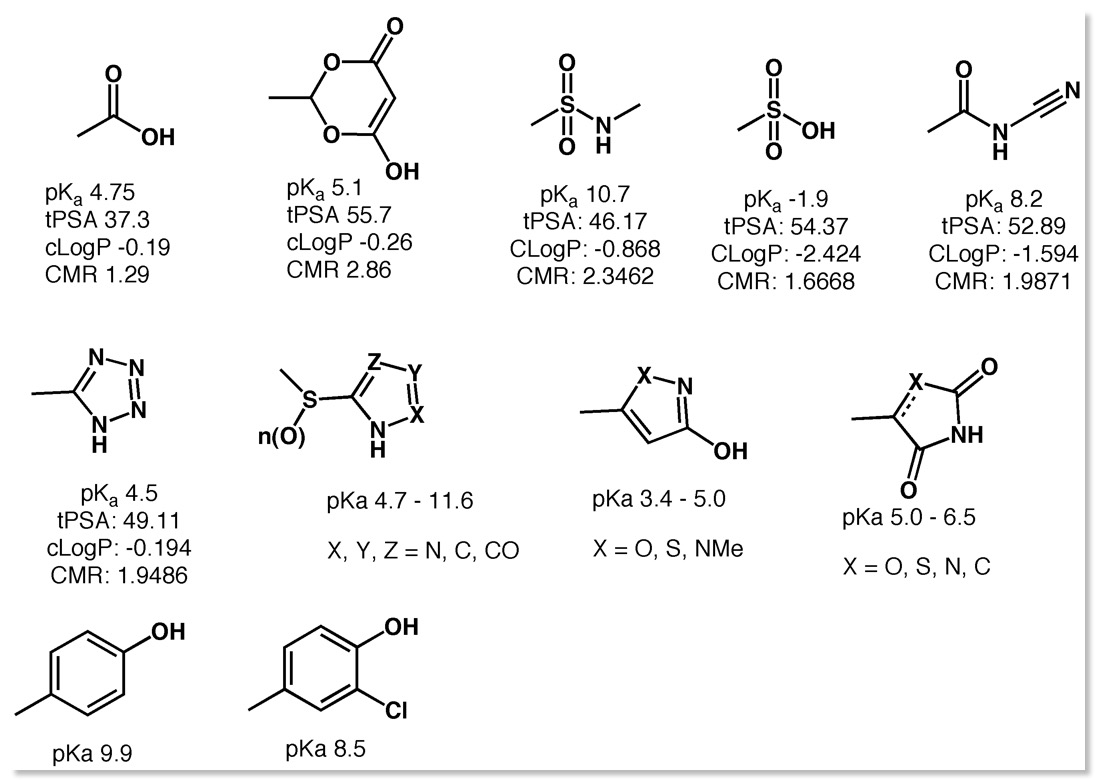
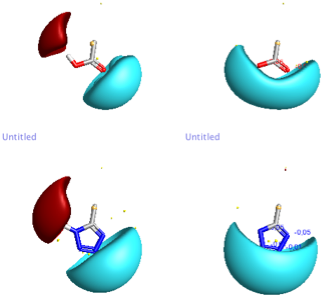
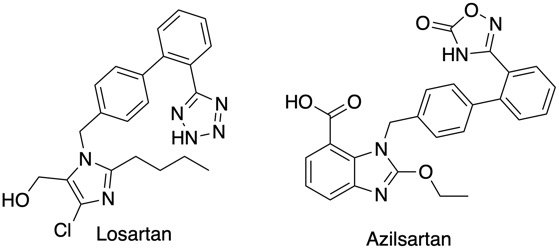
A frequently used bioisosteric modification in drug design is to replace a carboxylic acid group with 1H-tetrazole, as can be seen above both have similar pKa (4.5) and cLogP. A recent publication reviews the crystallographic data to compare the conformational preferences and intermolecular interactions of −COOH and the deprotonated −COO− with those of 1H-tetrazoles DOI in particular substituted phenyls as found in Losartan an angiotensin II receptor antagonist drug used mainly to treat hypertension. The carboxylic acid and tetrazole bioisosteric pairs exhibit very similar H-bond environments in crystal structures from the CSD, and the attractive energies of these H-bonds are very similar. However, the H-bond environments around 1H-tetrazole and tetrazolate substituents extend further away, by ≈1.2 Å, from the core of the connected molecule. Indeed an examination of the PDB confirms the need for this active site expansion to accommodate the tetrazole. The distribution of interactions found in the crystal structures is quite characteristic and is very well reproduced by using a tool like FieldView, the image below compares the carboxylic acid and carboxylate with the corresponding tetrazoles. Another angiotensin II receptor antagonist is Azilsartan which bears the bioisosteric oxadiazolinone (pka 6.1).
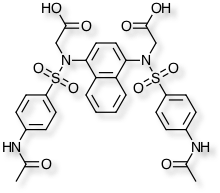
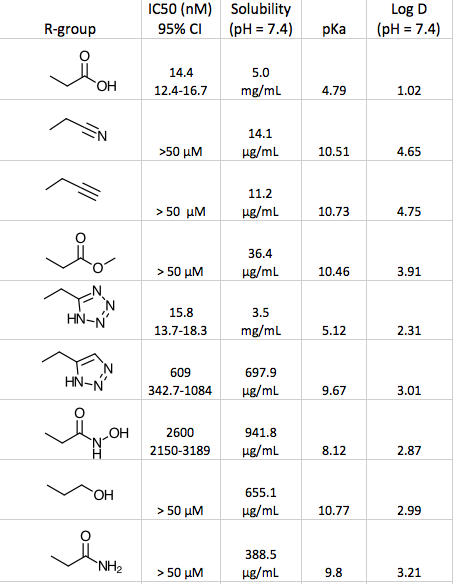
A paper describing work to inhibit the Keap1-Nrf2 protein-protein interaction explored bioisosteric replacements for a dicarboxylic acid lead. In addition to measuring biological activity they also compared solubility, pKa and LogD. DOI
It has been postulated that reactive acyl glucuronides may initiate toxicological or immune responses. To reduce the risk of metabolism-related liabilities associated with acyl glucuronides, tetrazoles have been used to replace carboxylates.
There is a very useful review http://dx.doi.org/10.1016/S0968-0896(02)00239-0 that describes several successful implementations of this particular bioisosteric replacement and also reviews the available synthetic methodologies.
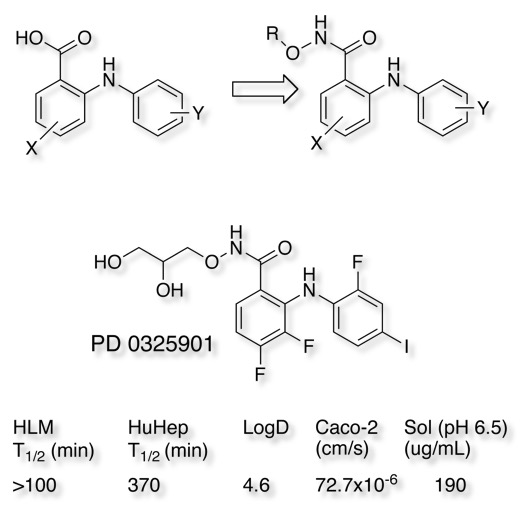
A recent publication Bioorganic & Medicinal Chemistry Letters 18 (2008) 6501–6504, identified Benzhydroxamic acid esters as excellent bioisosteric replacements for anthranilic acids, they speculate the esters are more resistant to metabolic processes within the cells such as hydrolysis and O-glucuronidation leading to a lower rate of metabolic degradation of parent compounds. The hydroxamic acid ester also has the option of modifying physicochemical properties. This work lead to the clinical candidate N-(2,3-dihydroxy-propoxy)-3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-benzamide PD 0325901.
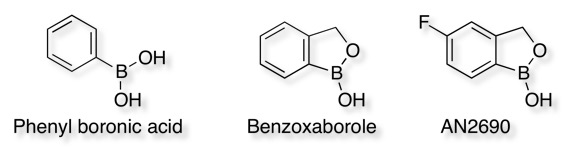
Recently there has been an interested in boronic acids and the analogous benzoxaboroles, the introduction of the oxaborole ring is to reduce the pKa from 8.8 to 7.3. More recently a study DOI dx.doi.org/10.1021/ml200215j has been published examining the substituent effects on the pKa of benzoxaboroles, as might be expected election-withdrawing substituents reduce the pKa to around 6, whilst electron-donating substituents increase the pKa to over 8. The 5-fluoro analogue being the novel topical ant-fungal agent AN2690.
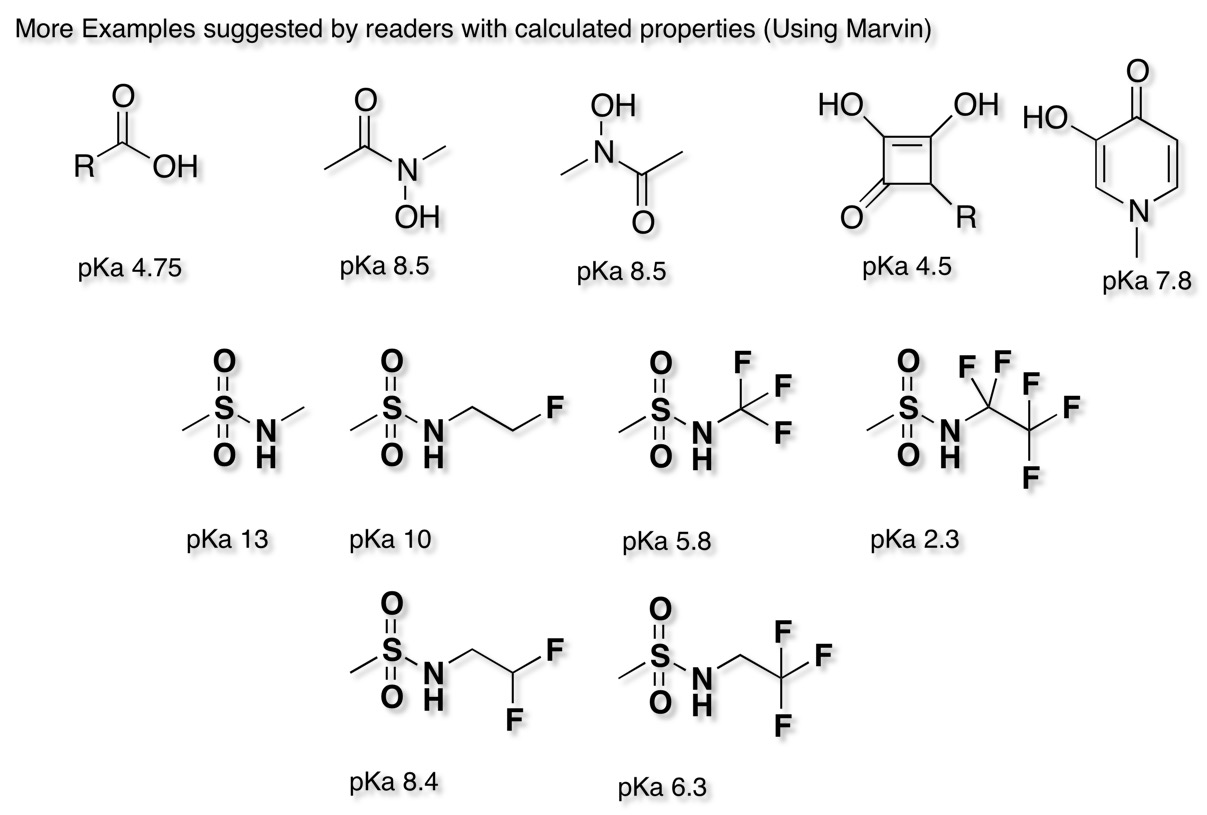
Five membered cyclic sulfonimidamide ring systems have also been explored DOI and their properties compared with the corresponding carboxylic acids and tetrazoles.
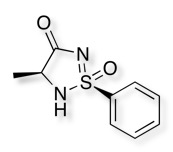
The cyclic sulfonimidamides were found to be less polar than the corresponding carboxylic acids and tetrazoles, were slightly less acidic may show improved cell penetration.
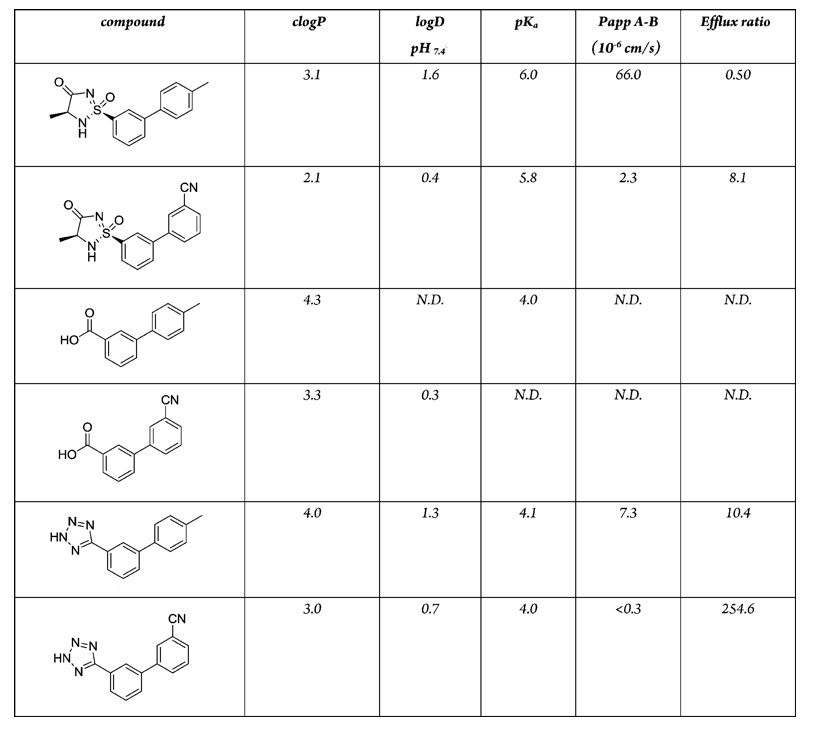
Aryl and Acylsulphonamides have been used as key acidic features in the pharmacophore of NaV1.7 Inhibitors DOI and DOI however the authors note significant challenges in attempting to optimise these series in particular, transporter-mediated clearance, CYP2C9 inhibition, TDI of CYP3A4, PXR activation, and numerous metabolic liabilities. The presence of an acidic functionality that can be fully ionized at physiological pH is a key interaction and studies have shown that this engages two arginine residues. A recent publication DOI describes the 1,2,4-Triazolsulfone (below) as a novel acidic bioisostere.
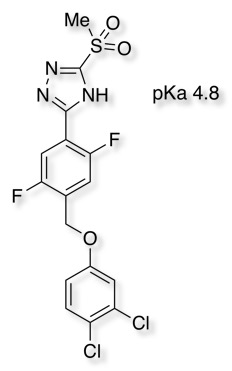
Subsequent optimisation identified analogues demonstrating high affinity, low clearance and good rat pharmacokinetics.
In order to overcome the disadvantages of carboxylic acid derivatives present in aldose reductase inhibitors researchers have successfully introduced the 2,6-difluorophenol and tetrazole rings as bioisosteres of the acetic acid moiety, in addition 1-hydroxypyrazole have now been shown to be excellent bioisosteric replacement DOI.
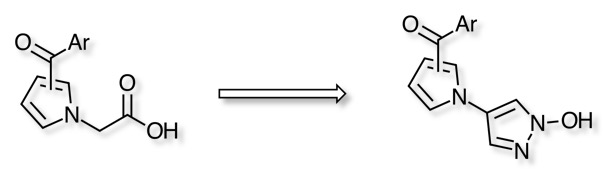
One advantage of the 1-hydroxypyrazole derivatives in comparison to the carboxylic acid and tetrazole is the higher pKa values, which can lead to more efficient tissue permeation.
In their work looking for Neprilysin inhibitors the Theravance group looked at acid bioisosteres for the known ligand LBQ657 which is the active component of the Prodrug Sacubitril. They replaced one of the carboxylic acids with the weakly acidic acyl triazole.
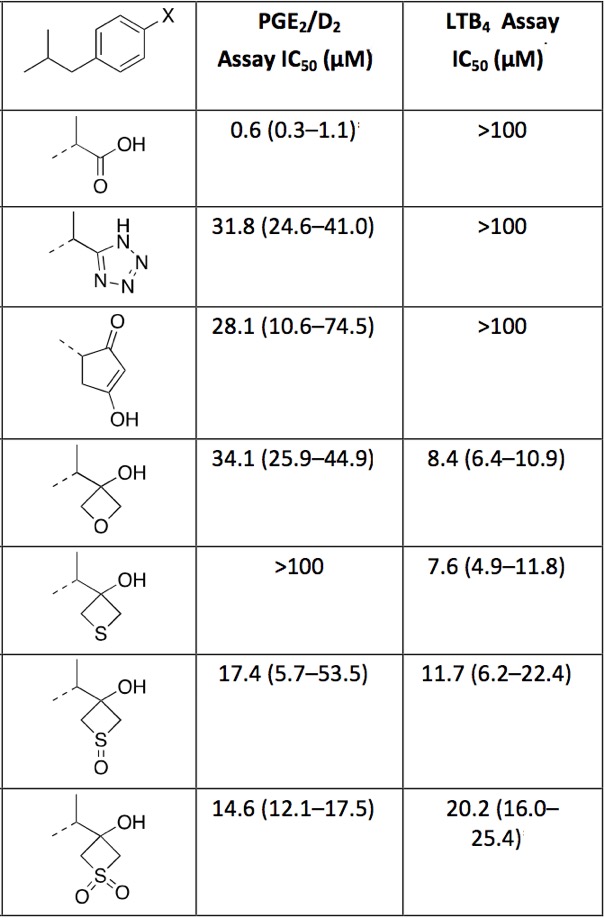
Since oxetanes have been used as carbonyl bioisosteres it is perhaps logical that Oxetan-3-ol, Thietan-3-ol and derivatives have been investigated as biososteres of a Carboxylic Acid DOI. Replacement of the carboxylic acid moiety of ibuprofen with the comparatively less acidic and more permeable four-membered ring structures results in analogs that were active in both enzyme and cell-based assays.
For phenol bioisosteres see the page on aromatic bioisosteres.
Worth Reading Carboxylic Acid (Bio)Isosteres in Drug Design DOI
Last Update 8 July 2022