Bioisosteric Replacements
Ester Bioisosteres
Need to adopt correct geometry and mimic potential H-bonding interactions
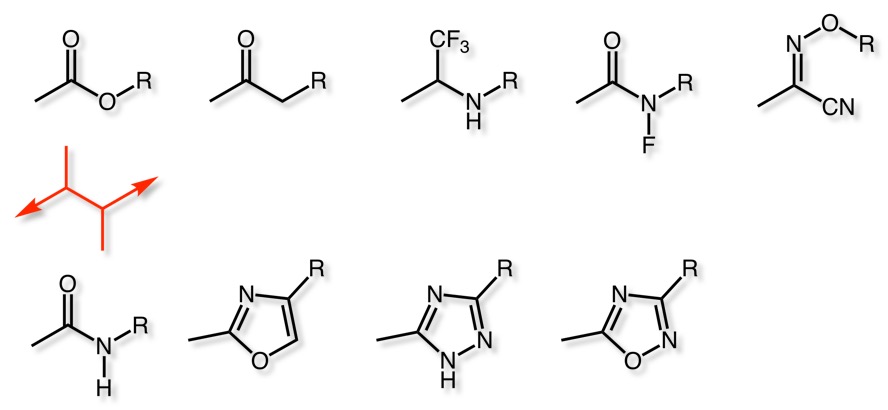
For examples of heterocycles as bioisosteric replacements for esters/amides see:
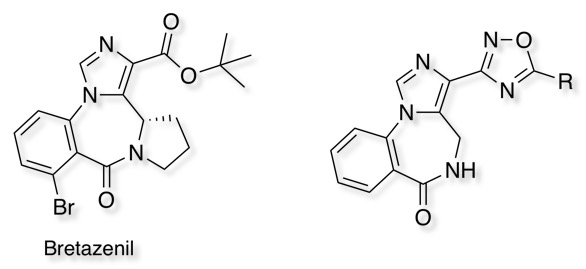
Benzodiazepine partial agonists J. Med. Chem. 1989,32, 2282
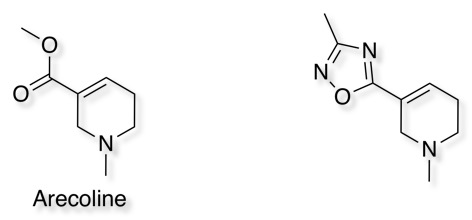
Muscarinic agonists J. Med. Chem., 1990, 33 (10), pp 2690–2697 DOI, Muscarinic agonists J. Med. Chem. 1997, 40, 4265
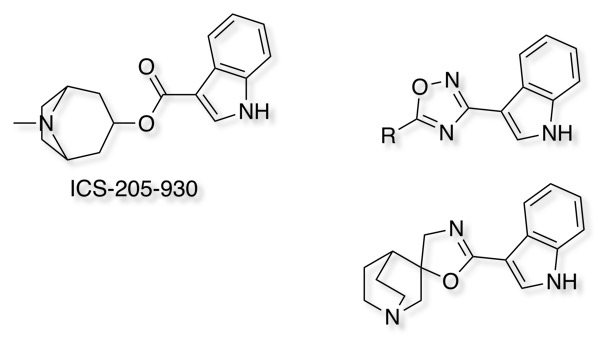
5-HT3 antagonists J. Med. Chem., 1991, 34 (1), pp 140–151 DOI and Tetrahedron Letters Volume 31, Issue 17, 1990, Pages 2445-2448 DOI
Store-operated calcium entry (SOCE) is a pivotal mechanism in calcium homeostasis, bioisosteric replacement of a labile ester with an oxadiazole afforded a class of modulators with high metabolic stability. DOI.
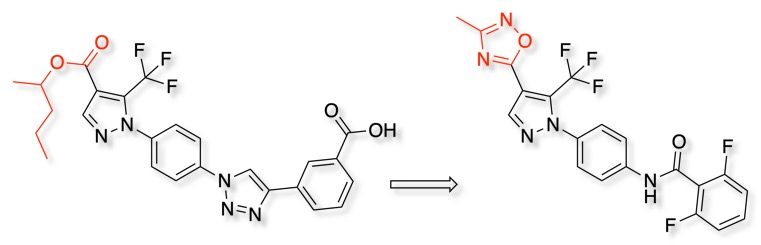
The image below shows the electrostatic surfaces generated using MOE.
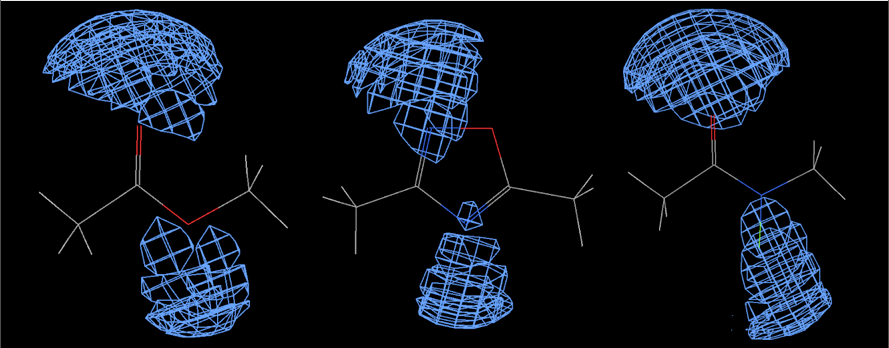
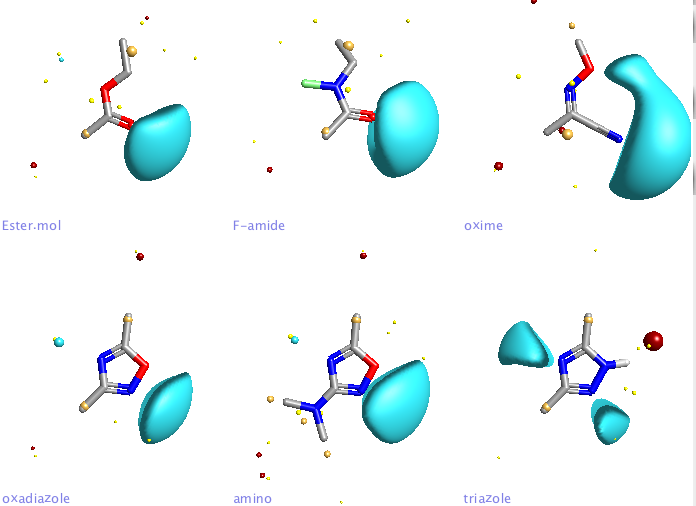
The image below shows the field surfaces generated using FieldView, this analysis also demonstrates the influence the substituent can have on the oxadiazole, the electron-donating -NMe2 increasing the size of the negative potential, potentially increasing the strength of any hydrogen bond. The physicochemical properties of the isomeric substituted oxadiazoles has been investigated, as might be expected increasing the H-bonding ability reduces LogP and solubility.
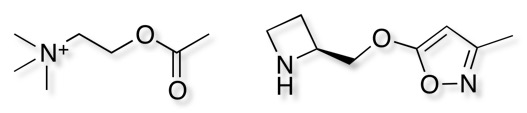
A series of nAChR ligands based on an isoxazole-ether scaffold as a bioisosteric replacement for the acetyl group of acetylcholine have been designed. Early stage absorption, distribution, metabolism, excretion, and toxicity studies also suggested favourable drug-like properties DOI.
Amide Bioisosteres
Amides are extremely common in both virtual and physical screening collections, amide bond formation is ax extremely reliable reaction and it provides an easy route to increasing diversity. The amide functional group is enzymatically very labile in vivo; therefore, amide bond bioisosteres that improve metabolic stability are of great interest. Amides can exist as "cis" or "trans" amides and the one of the key opportunities for bioisosteric replacement is to use a ring system to freeze one isomer by the use of a ring system. Indeed, these ring systems allow the exploration of a variety of different vectors. Other bioisosteres that don't include a ring system include ester, carbabamates and ureas.
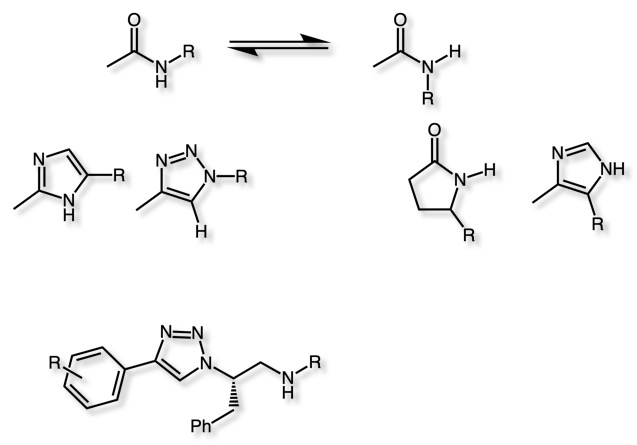
The 1,2,3 triazole is an increasingly commonly used bioisosteric replacement for amide, it is relatively easy to synthesise via copper catalysed reaction of an azide with an alkyne. The 1,2,3-triazole unit is resistant to cleavage mediated by proteases, oxidation, and hydrolysis DOI. Triazole-linked reduced amide isosteres DOI easy prepared using “click” chemistry as potential BACE1 inhibittors.
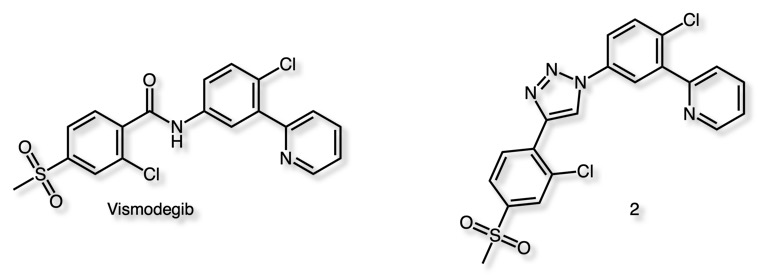
Vismodegib is a drug for the treatment of basal-cell carcinoma (BCC) is a smoothened antagonist and was the first Hedgehog signaling pathway targeting agent. Compound 2 possessing the 1,2,3-triazole ring in place of the amide bond (17) displayed an appreciable activity benefit in HMEC-1 cells (IC50 = 9.6 ± 0.7 μM) as compared to Visodegib (IC50 = 41 ± 3 μM).
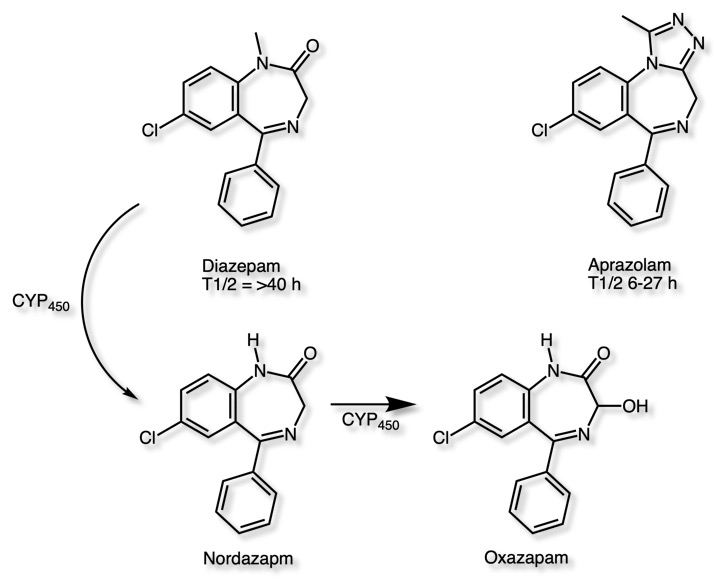
Diazepam is undergoes CYP450 mediated methylation and oxidation to yield long-acting and metabolically active metabolites with extended half-lives (half-life of 50−120 h). In contrast, studies in human indicated that Alprazolam did not produce active metabolites, resulting in significant suppression of diazepam-induced toxicity.
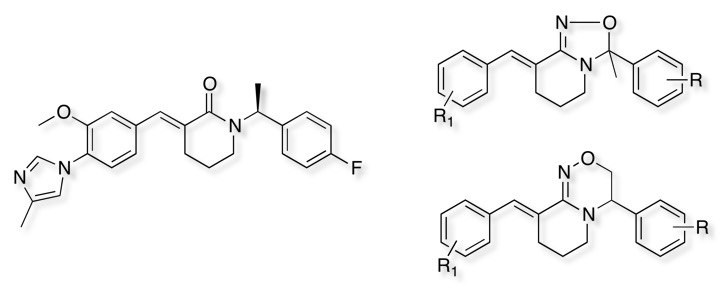
Oxadiazolines and Oxadiazines have been shown to be excellent bioisosteric replacements for an amide yielding a series of potent and Highly Efficacious γ-Secretase Modulators in Vivo DOI. Compounds from these series are well absorbed in rat, dog and monkey (F = 40-100%) and are not PGP substrates in vitro. In contrast to the amide the oxadiazolines and oxadiazines were found to be weaky basic allowing salt formation.
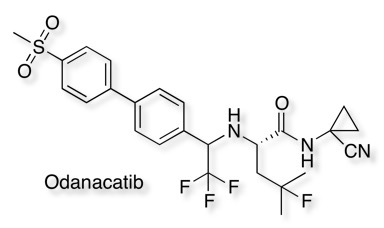
The replacement of the amide carbonyl by CF3 has been demonstrated in the development of Odanacatib (MK-0822) an inhibitor of cathepsin K, it should be noted however that the bioisosteric transformation in it’s self did not improve metabolic stability.
CGRP is a popular target for pain DOI and as might be expected a number of the leads resemble modified peptides, work by BMS has shown that pyridyl can act as an amide bioisostere with improved membrane permeability DOI.
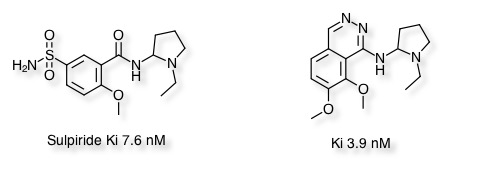
A recent publication by Wermuth (DOI: 10.1039/c1md00074h) describes pyridazines as aromatic bioisosteres, interestingly the pyridazine ring has also served as an amide biosiosteric replacement DOI to mimic the the D2 antagonist Sulpiride.
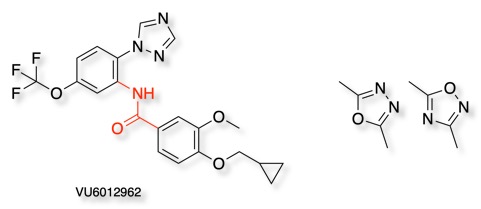
VU6012962 is a mGlu7 negative allosteric modulator (NAM), but metabolic instability of the amide linker has limited in vivo studies. In an effort to improve metabolic stability an array of 12 bioisosteric heterocycles were prepared DOI. Whilst many of the replacements proved to be inactive, the 1,3,4- and 1,2,4-oxadiazoles retained useful activity. Both NAMs displayed favourable profiles, with predicted hepatic clearances (CLhep) in rat of around 27 mL/min/kg and modest plasma protein binding.
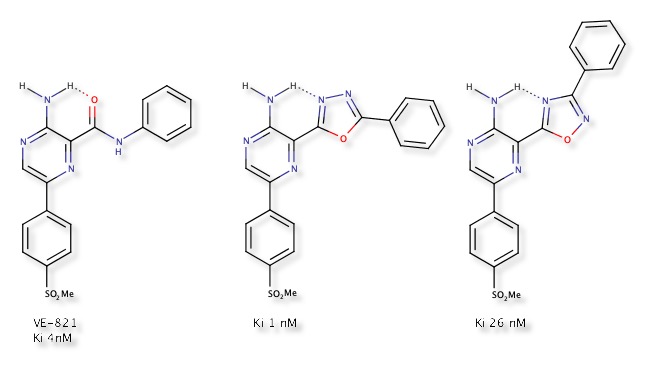
VE-821 is an ataxia telangiectasia mutated and rad3-related (ATR) kinase inhibitor Ki 4 nM DOI, these enzymes work together to support DNA damage and repair. ATR inhibition has been shown to enhance the toxicity of DNA damaging chemotherapy to many cancer cells in multiple preclinical studies, while healthy tissue with functional ATM can tolerate ATR inhibition. Significant effort was focussed on replacing the amide linkage with a variety of heterocycles, a key observation was a proposed intramolecular hydrogen bonding interaction between the 2-aminopyrazine and the amide carbonyl.
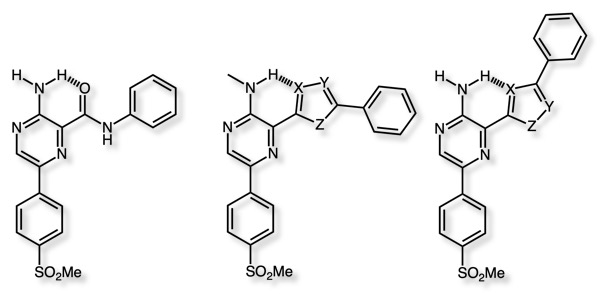
The preference for the alternative conformation of the 1,2,4-oxadiazole was predicted by the positive relative conformational energy, and is in agreement with the observation that aromatic nitrogen atoms are better H-bond acceptors than aromatic oxygens, in contrast the 1,3,4-oxadiazole improved affinity.
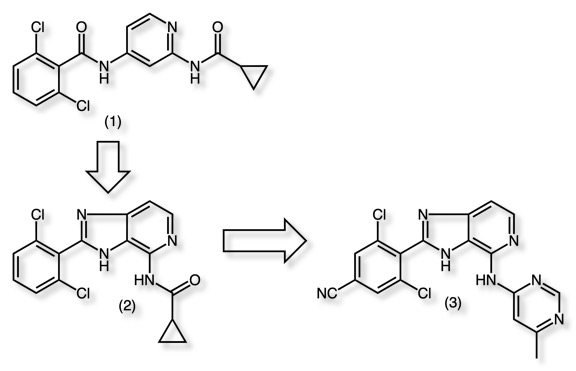
A nice example of the use of heterocycles to replace amides is found in the identification of selective TYK2 inhibitors DOI. The initial lead actually contains two amides (1), one is replaced by the annulated imidazole (2) followed by subsequent replacement of the second amide by a pyrimidine (3).
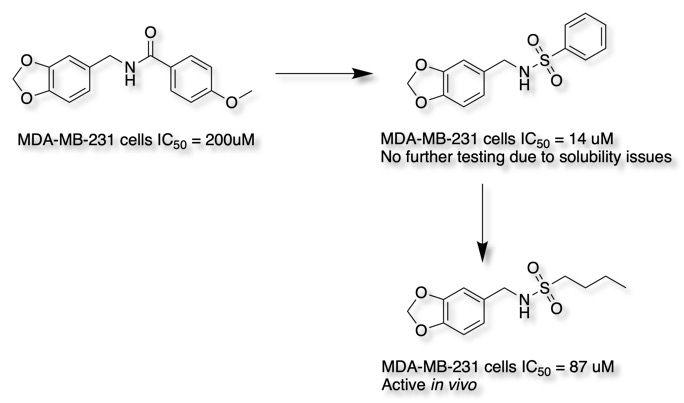
Sulphonamides have been used as bioisosteric replacements for amides, however it should be noted that in some cases whilst metabolic stability may be improved this can cause solubility issues DOI.
Carbamate Bioisosteres
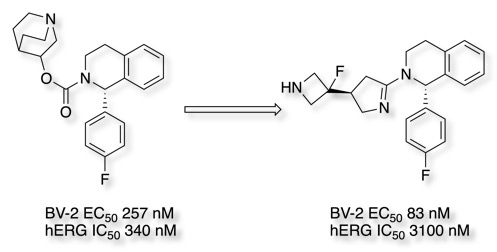
Five membered rings have also been used as carbamate bioisosteres DOI, the conformationally restricted carbamate isosteres led to the identification of compound with a 3-fold improvement in BV-2 potency and excellent mouse bioavailability F = 99%.
Worth reading
Applications of Amide Isosteres in Medicinal Chemistry DOI.
Surveying heterocycles as amide bioisosteres within a series of mGlu7 NAMs: discovery of VU6019278 DOI.
Amide Bond Bioisosteres: Strategies, Synthesis, and Successes DOI
Last Update 16 Feb 24