Solubility
Poor solubility can have an impact on various stages of the drug discovery process, whist perhaps the most important is the impact poor solubility can have on gastrointestinal absorption it may also preclude other routes of administration (intravenous), in some cases this can be addressed by Formulation. However complex formulations can have a significant effect on the development cost and timelines. Many companies categorise compounds based on the Biopharmaceutics Classification System regarding solubility and permeability. A drug is considered highly soluble when the highest dose strength is soluble in 250ml or less of aqueous media over the ph range of 1 to 7.5. The volume estimate of 250ml is derived from typical bioequivalence study protocols that prescribe administration of a drug product to fasting human volunteers with a glass of water.
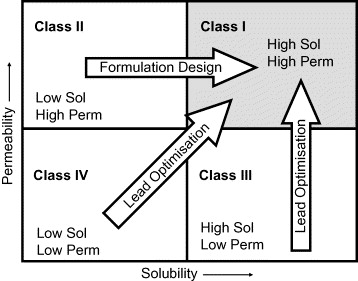
However it is worth noting that formulation can do little improve the absorption of Class IV and Class III compounds which are compromised by poor membrane penetration.
Solubility may also have an impact on preclinical assays, limited solubility in preclinical ADMET assays may give a false impression of the compounds profile in in vitro assays. Many of the false positives seen in Fragment-based screening are thought to be due to poor solubility at the high concentrations used in the screen.
Other concern with poorly soluble compounds is that solubility might be further compromised if a new crystalline form is discovered during development.
Assays
Solubility can be measured in either Kinetic or Equilibrium Methods.
Kinetic assays usually involve diluting a DMSO solution with aqueous buffer, turbidity is measured using UV-vis spectrophotometry at 620 nM. These assays can be carried out in multi-well format and can thus be part of a screening cascade, and the conditions are easily modified to explore the influence of temperature or pH of the aqueous buffer. A citrate buffer pH2 is usually used to mimic the conditions found in the stomach. The data is useful for relative ranking but should not be regarded as an absolute figure. Coloured compounds can cause interference. This information has been particularly useful in the design of fragment libraries where solubility at the high assay concentrations is critical.
Another issue that can arise in high-throughput screening is aggregation-based artefacts, a confocal Static Light Scattering plate reader assay can be used to assess the critical aggregation concentration (CAC). This method achieves sensitivities at sub-micromolar compound concentrations, enabling a reliable estimate of compound monomer solubility. The subject of aggregation is assays has been extensively studied.
Worth reading
High throughput solubility measurement in drug discovery and development DOI
Solubility at the Molecular Level: Development of a Critical Aggregation Concentration (CAC) Assay for Estimating Compound Monomer Solubility DOI
Equilibrium assays require large amounts of compound and can be very time-consuming, they usually involve preparation of a saturated solution (stirred 24 hours) followed by filtration and HPLC analysis. The results are more accurate but are usually only determined as part of the development program for a candidate compound.
Tip: Ask for the samples back, you can recover grams of material.
Last Updated 11 November 2017