Separation of PK and PD
For indications for which require an extended pharmacological profile a compound with a long binding half-life can have a duration of action which extends beyond the presence of drug levels in plasma needed for biological activity. In particular it may be possible to extend duration of action at the intended target whilst limiting activity at off-target proteins. Whilst this could be achieved by irreversible covalent binding there is now a growing body of evidence that many small molecules can display slow off rate kinetics. It is worth noting at this point that the separation between PK and PD may also be the result of active metabolites.
The situation was elegantly summarised in a publication from Copeland et al. DOI
A dominant assumption in pharmacology throughout the 20th century has been that in vivo target occupancy-and attendant pharmacodynamics-depends on the systemic concentration of drug relative to the equilibrium dissociation constant for the drug-target complex. In turn, the duration of pharmacodynamics is temporally linked to the systemic pharmacokinetics of the drug. Yet, there are many examples of drugs for which pharmacodynamic effect endures long after the systemic concentration of a drug has waned to (equilibrium) insignificant levels. To reconcile such data, the drug-target residence time model was formulated, positing that it is the lifetime (or residence time) of the binary drug-target complex, and not its equilibrium affinity per se, that determines the extent and duration of drug pharmacodynamics.
Information about binding kinetics can be obtained from both radioligand binding and functional studies Med. Chem. Commun., 2012, 3, 645–651 but it should be noted that temperature can have a significant influence on the kinetics and experiments are often not carried out at a physiological 37degrees C.
If we simply compare the affinity of two compounds with similar nanomolar affinity it is not possible to determine the off-rate from such a determination.
Drug A, Kd 1 x 10-9, Kon 109, Koff 1 (Very Fast onset and off rate)
Drug B, Kd 1 x 10-9, Kon 105, Koff 0.0001 (Very slow onset and off rate)
It is worth noting that the difffusional upper limit for two molecules colliding in aqueous solution, a bimolecular process, is 109M-1s-1. As a rule of thumb, kon values for proteins and ligands are in the 106 to 107 M-1s-1 range so complexes with KD in the 10-6 to 10-7M range will have lifetimes somewhere near one second.
The avidin-biotin complex has a koff ≤ 10-6sec. To convert first order rate constants into half lives, use the simple relationship
t1/2 = ln2/k = 0.693/k
So for the avidin-biotin complex
t1/2= 0.693/0.000001 = approx 80 days.
If we compare data from a range of muscarinic M3 antagonist in the table below it is clear that the reason Tiotropium displays long lasting pharmacological effects despite very low trough levels in the clinic is due to the off-rate from the M3 receptor. In addition to long duration of action, the ability to retain effectiveness at lower blood levels results in an enhanced therapeutic window. Since Tiotropium has a faster dissociation rate from M1 and M2 muscarinic receptors this results in improved cardiovascular safety.
| M3 antagonists | KD/KI | ON RATE X109M-1 MIN-1 | OFF-Rate MIN-1 |
|---|---|---|---|
| Atropium | 0.2 nM | 1.5 | 0.27 (2 min) |
| Ipatropium | 0.2 nM | 0.5 | 0.07 (10 min) |
| Clidinium | 0.3 nM | 10 | 0.02 (30 min) |
| Tiotropium | 0.8 pM | 0.16 | 0.0015 (34.7 h) |
Evaluation of target kinetics
Ideally it would be useful to be able to identify those structure classes that exhibit slow dissociation kinetics, however we can’t get that sort of information from simple screens for affinity. A number of technologies are available:-
Surface Plasmon Resonance (eg Biacore), cost of datapoint $2, flow cell based, 200 data points per day.
Radioligand, Cost depends on radioligand, microtitre plate, capacity depends on degree of automation
Biolayer interferometry, cost of datapoint $2, capacity ?
Functional assays, cost is variable, low capacity
Properties of molecules exhibiting slow dissociation kinetics
KD = koff (s-1)/kon (M-1s-1)
Cannot improve association rate constant above ~10‐8 M‐1s‐1 (diffusion control), however the dissociation rate constant can be infinite (ie covalent binding!)
Miller et al Med. Chem. Commun., 2012, 3, 449–452 have tried to identify the physicochemical properties associated with extended target engagement. Compounds with an Koff of less than 15 min were defined as ‘‘rapid offset’’, whilst the ‘‘slow offset’’ bin was defined as compounds having a dissociation half-life of >2 h. The ‘‘moderate offset’’ bin contained compounds with a dissociation half-life from 15 min to 2 h.
Compounds exhibiting slow dissociation kinetics were more lipophilic, higher molecular weight with a greater number of rotatable bonds.
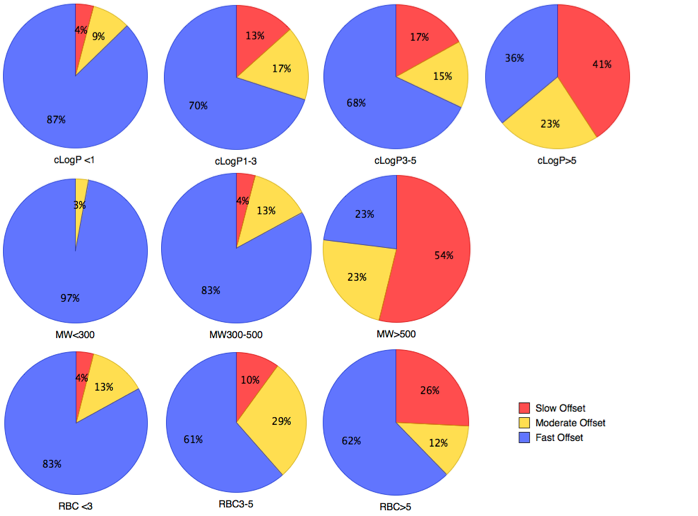
There has to be some care in the interpretation of these observations, it is likely that larger molecules will have a greater number of rotatable bonds and increased lipophilicity. Interestingly ionisation state may have an influence on slow dissociation, with molecules containing an acidic group displaying extended duration.
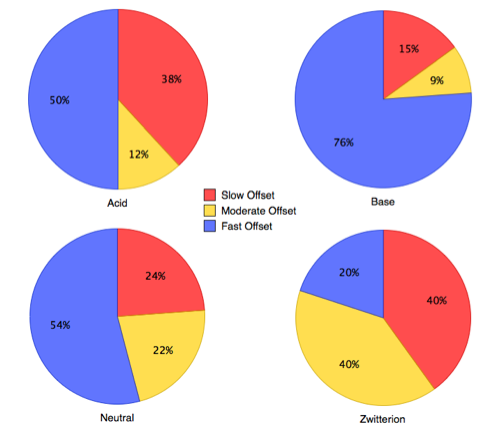
Whilst these properties may not be ideal for an oral drug, the increased target occupancy may overcome any PK liability, in addition, they may be ideal for other routes of administration.
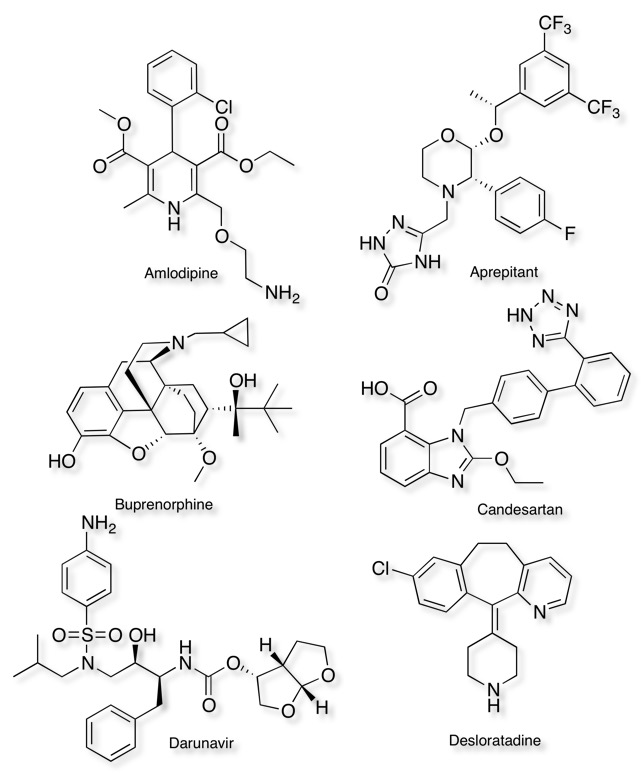
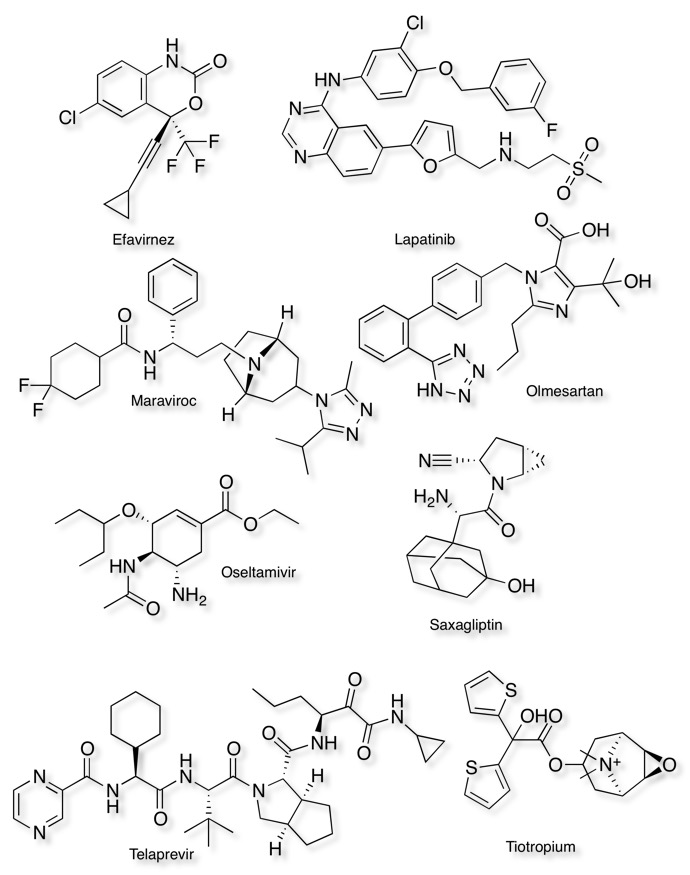
Examples of compounds with slow dissociation kinetics
Name, (T1/2), target, Indication
Amlodipine (77 min) Calcium channel blocker, hypertension
Aprepitant (154 min) NK1 antagonist, cancer therapy induced emesis
Buprenorphine (166 min) partial agonist at mu and kappa opioid receptors and as an antagonist at delta receptors. , pain
Candesartan (11.5 h) angiotensin II receptor antagonist, hypertension
Darunavir (>240 h) HIV aspartyl protease inhibitor, antiviral
Desloratadine (>6 h) H1 antagonist, antihistamine
Efavirnez (4.1 h) non-nucleoside reverse transcriptase inhibitor, antiviral
Lapatinib (300 min) growth factor inhibitor, anticancer
Maraviroc (10.5 h) CCR5 antagonist, antiviral
Olmesartan (72 min) angiotensin II receptor antagonists, hypertension
Oseltamivir (60 min) neuraminidase inhibitor, antiviral
Saxagliptin (5.1 h) DPP-4 inhibitor, diabetes
Telaprevir (2.9 h) HCV NS3-4A serine protease inhibitor, HCV
Tiotropium (34.7 h) muscarinic receptor antagonist, COPD
Worth Reading
The importance of binding kinetics and drug-target residence time in pharmacology DOI
The need for high throughput kinetics early in the drug discovery process DOI
Investigation of the effect of molecular properties on the binding kinetics of a ligand to its biological target DOI
Last updated 10 November 2023