Metal Chelation in Enzyme Active Sites
A hard ion like magnesium is almost always octahedrally coordinated predominantly to oxygen.
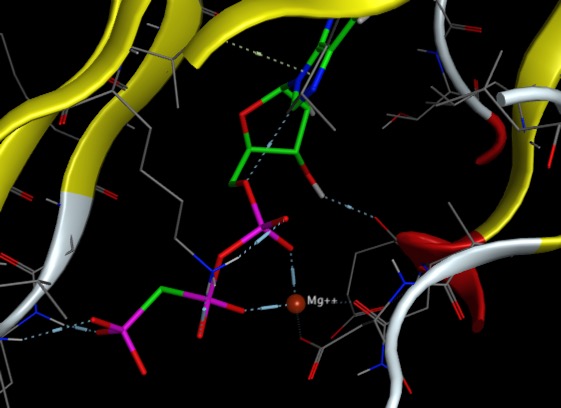
If you see an octahedrally coordinated 'water' in a protein crystal structure, you can bet it's really a magnesium. Magnesium is sometimes involved in hydrolytic reactions. This is common when phosphate groups are involved, probably because the affinity of Mg2+ for phosphate groups is high. In the image below of human protein kinase Aurora A (PDB ID 6CPF), the magnesium coordinates with Asp274 and Asn261 and the phosphate oxygens of the adenine triphosphate.
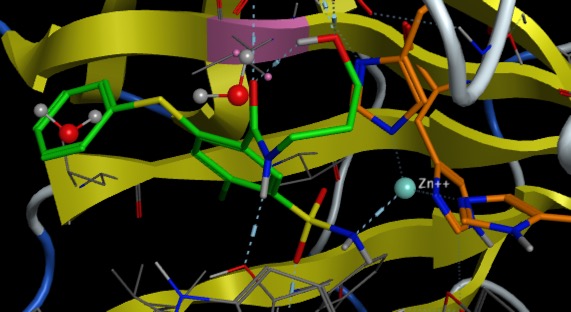
Soft ions like zinc tend to have less rigid (4- or 5-) coordination and displays high affinity for nitrogen and oxygen donor atoms as well as for sulhur. It is therefore found to be bound to histidine, the carboxylates of glutamate or aspartate, and cysteine. When the zinc has a catalytic role, it is exposed to solvent, and generally one water molecule completes the coordination, in which case the coordinating amino-acids are usually histidines. In the image below (PDB ID 6QUT) the active site of carbonic anhydrase the zinc is coordinated to 3 histidines (shown in orange) and the sulphonamide of the inhibitor (green).
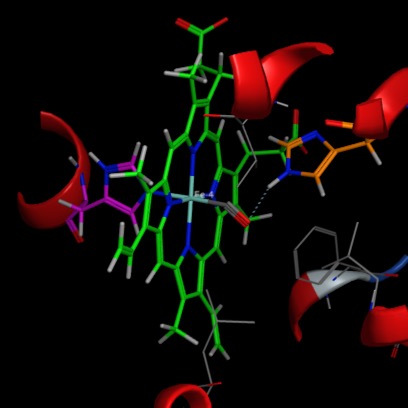
Iron can take various oxidation states and thus coordination states snd may change oxidation state during reaction. Most organisms require molecular oxygen in order to survive and evolved a variety of dioxygen-carrier proteins, the best known of which is haemoglobin. In haemoglobin four of the five ligands that make up the active site are provided by a square-planar tetradentate ligand, the protoporphyrin. An additional axial ligand is an imidazole from a histidine residue provided by the protein, and the remaining sixth coordination site is available for the exogenous ligand, oxygen. In the image below (PDB ID 6KYE), the porphyry ring is shown in green, His87 (purple) takes up one of the axial coordination sites while cabot monoxide takes the position normally occupied by oxygen.
A similar arrangement is in the Cytochrome p450 enzymes.
Worth reading:- Structural and Functional Aspects of Metal Sites in Biology R Holm, P Kennepohl, and E I Solomon Chem. Rev. 1996, 96, 2239-2314
Examples of Magnesium ligands
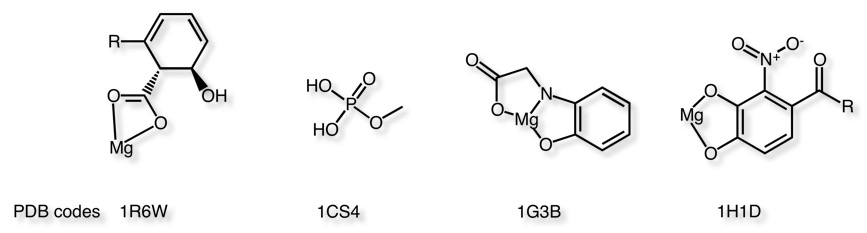
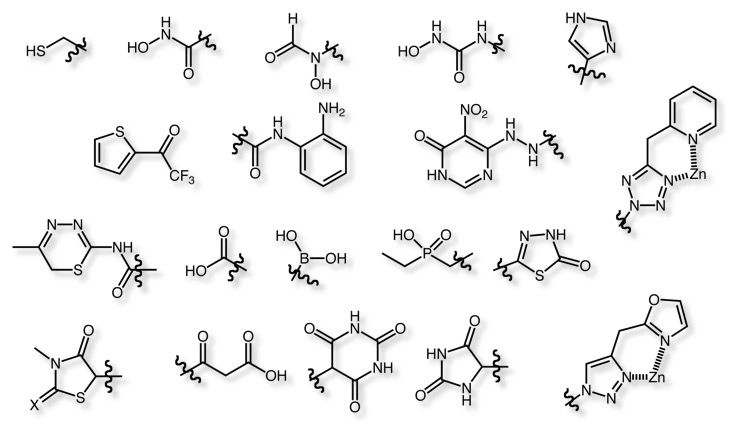
Examples of Zinc ligands
There are a wide variety of well-characterised zinc proteins, including the Cu-Zn superoxide dismutases (other forms have Fe or Mn), carbonic anhydrase, an abundant protein in red blood cells responsible for maintaining the pH of the blood. At least five distinct CA families and these families have no significant amino acid sequence similarity and in most cases are thought to be an example of convergent evolution. In addition, there are alcohol dehydrogenase, and a variety of hydrolases involved in the metabolism of sugars, proteins, and nucleic acids.
The zinc metalloenzymes provide a number of important therapeutic targets and as you might expect a wide variety of functional groups have been identified that bind to the zinc.
Examples of Iron ligands
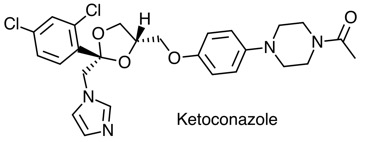
Many diverse known inhibitors are known, and they often include a heterocycle that binds to the haem iron in the active site, for example the anti-fungal ketoconazole.
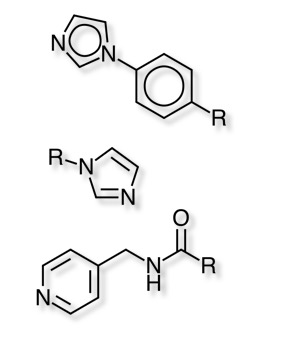
See also Metalloprotease Inhibitors
Last updated 4 April 2020