Tuning the basicity of amines
Tuning the basicity of amines
Whilst basic centers are attractive features to have in drug molecules because they offer the ability to create water soluble, crystalline, high melting salts, usually without the plasma protein binding associated with carboxylic acids. They can cause problems with HERG, CYP450 2D6.
However it is possible to tune the pKa of the basic center and thus modulate the properties of the molecule. Whilst primary and secondary amines are more basic than tertiary increasing alkyl substitution does usually increase basicity.
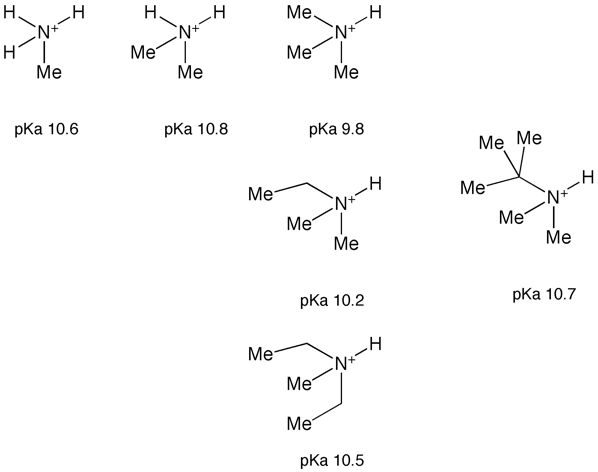
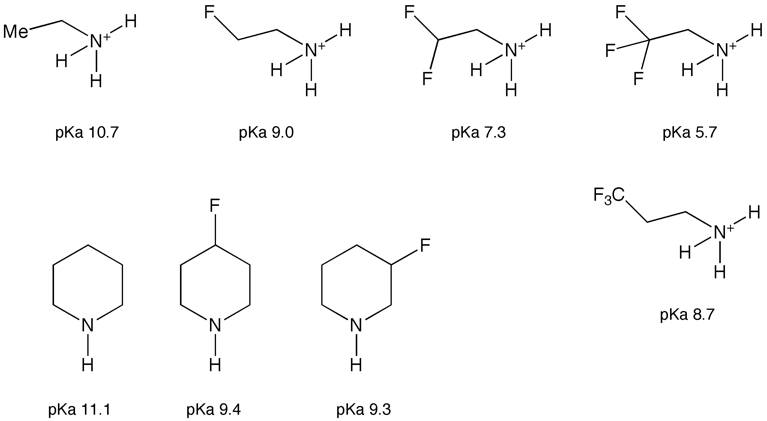
Usually however you will be looking to reduce basicity, this is best achieved by heteroatom substitution, in particular by replacing a hydrogen with fluorine since this will have little steric impact. The chart below shows the impact of adding fluorine atoms the influence of which can be observed through several bonds. Similar effects can be seen in cyclic amines, however the effect is influenced by the conformation of the ring with the equatorial fluorine having the greater effect. It should be noted that these changes may have a detrimental effect of lipophilicity.
The influence of fluorine's impact on pKa and in vitro PGP-mediated efflux for a series of PDE9 inhibitors (below) has been explored DOI. Introduction of fluorine impacted both the basic amine but also the acidity of the lactam. Comparisons through the series of compounds and their fluorinated analogues reveals a systematic decrease in acidic pKa between 1.2–1.7 (pKa (acidic)), and shifts for the basic pyrrolidines between 1.1–3.3 units (pka basic). Introduction of F into the NH lactams often served to increase PGP activity, in contrast introduction into the N-Methyl lactams reduced PGP activity.
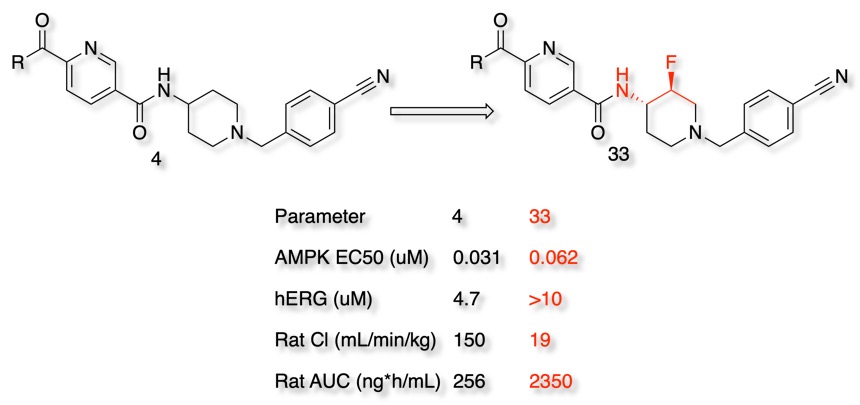
Modulation of the pKa of a basic amine can have multiple beneficial effects. In the example below DOI reducing the basicity of the piperidine nitrogen reduces hERG activity and also reduces clearance.
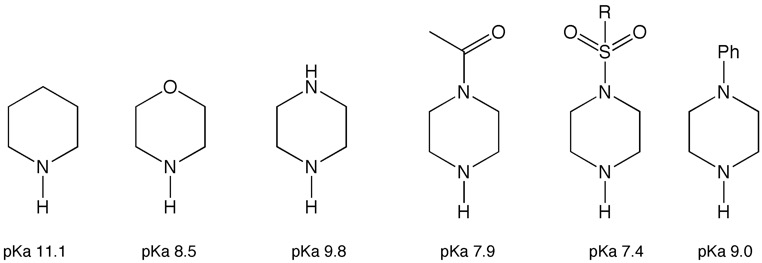
The basicity of the piperidine nitrogen can also be modulated by moving to the morpholine or N-substituted piperazine, particularly when the nitrogen is substituted by acyl or sulphonyl groups. Similar effects can be seen in acyclic systems where a beta hydroxy or alcoxy group will reduce the pka by 1.2 units, and similar substituents at the gamma position by -0.8 units.
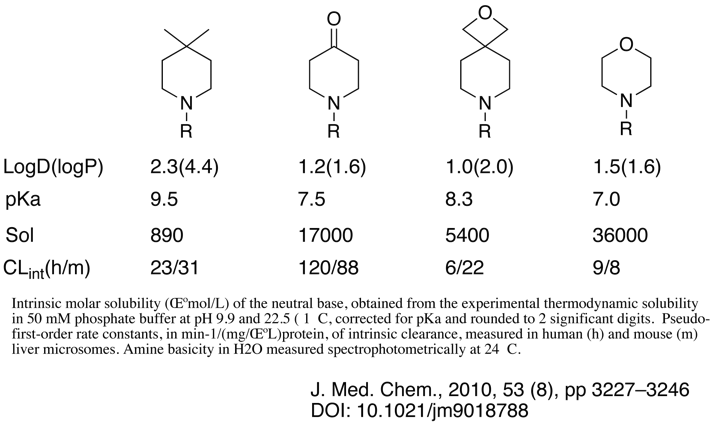
Strongly electron-withdrawing groups like nitriles, esters, amides, ketones, oxetanes and polyheteroatom hetrocycles e.g. oxadiazoles, can also be used to modulate pKa but they do introduce further pharmacophoric elements. An interesting paper by Wuitschik et al looks at oxetanes as replacements for carbonyl and how they might influence physicochemical properties, whilst a variety of different structures are compared I’ve just pulled out the data for 4-substituted piperidines. Whilst the influence on pKa is intermediate between piperidine and the corresponding 4-piperidone the more polar oxetane means that the resulting LogD is actually lower.
Last Update 14 Dec 2023