Absorption and Bioavailability
Deciding whether a drug is absorbed by Passive or Active-Transport
Passive transport
- Not concentration dependent (non-saturable), unless high concentration disrupt membranes.
- Not subject to inhibition
- Less cell type specific than carrier mediated transport
- Less structure specific than carrier-mediated transport; there is a general dependence on lipophilicity for structurally diverse compounds, however the relationship with LogD is non-linear with an optimum of LogD 1-2. I’ve added the human bioavailabilities to scatter plot (generated using Vortex), whilst in general those with poor membrane permeability have low bioavailability you should remember that metabolism can reduce the bioavailability of even the best absorbed compounds.
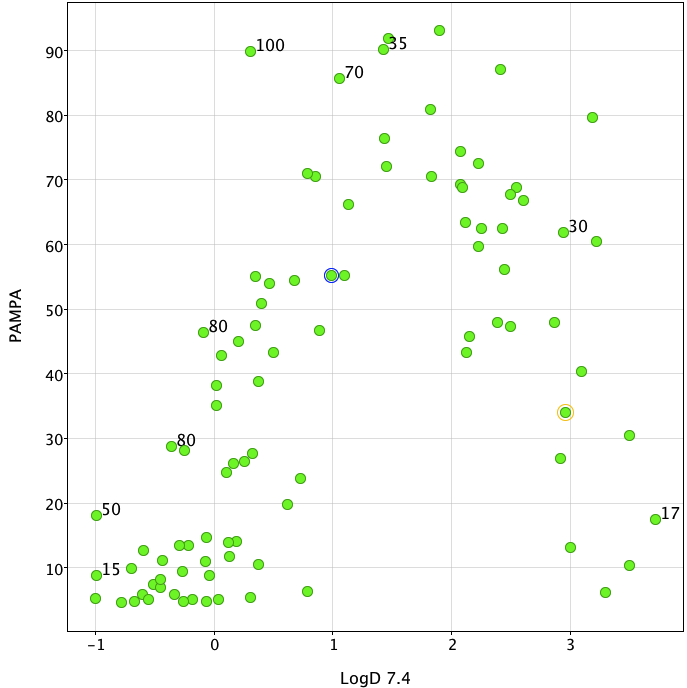
Data taken from (Kansy, M. et al. in Pharmacokinetic Optimization in Drug Research: Biological, Physicochemical and Computational Strategies (eds Testa, B., Van de Waterbeemd, H., Folkers, G. & Guy, R.) 447-464 (Wiley-VCH, Zurich, 2001).)
Carrier-mediated transport
- Concentration dependent (saturable)
- However some transporters such as glucose or amino acid transporters have very high capacities
- Subject to inhibition
- More structure specific than passive transport, but dependence on lipophilicity could be identified in a narrow chemical series
- Cell type specific; requires expression of the transporter
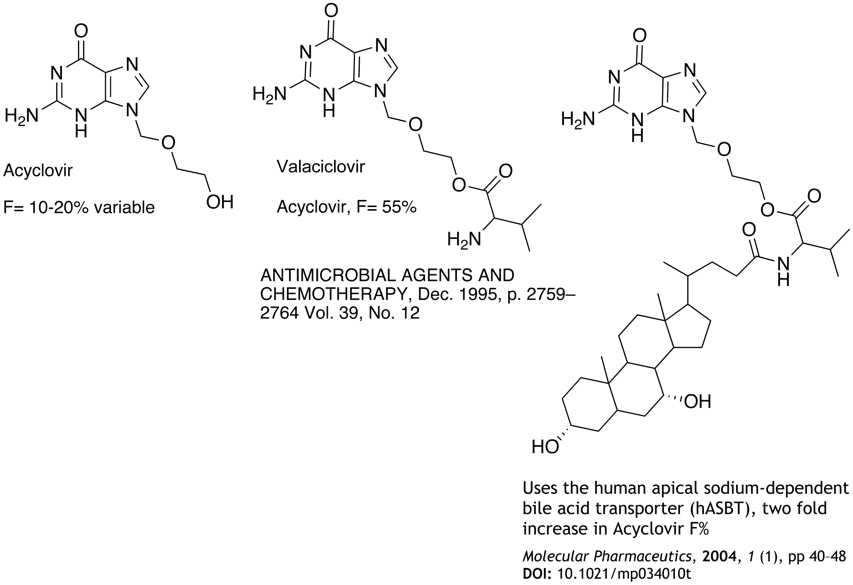
If the absorption is permeability limited Possibly due to efflux pump Can it be saturated at reasonable doses? If the absorption is solubility limited Look at formulations Poor solubility could be a liability in development, particularly if a new less soluble crystal form is identified. Improve solubility Reduce molecular weight, introduce ionisable groups, H-bonding groups, reduce LogP Prodrugs, Acyclovir is very poorly absorbed and a number of prodrugs have been developed to improve absorption and hence bioavailability. The examples below use active transport systems to increase uptake.
Assays
PAMPA
- Rapid screen using artificial hexadecane membranes
- High-throughput
- No need to grow cells
- No transporters
- Caco-2
- Monolayers of human intestinal cell
- Transepithelial electrical resistance >1000 ohms as index of monolayer integrity.
- Papp > 50 nm/s high permeability (Propanolol >200)
- Papp 10-50 nm/s medium
- Papp <10 low (Cimetidine <10)
MDCK-hMDR1
- Canine kidney cells transfected with hMDR1 gene, also used as model of blood brain barrier because of high level PGP expression.
- Measure permeability both ways across membrane A-B and B-A.
- Papp > 150 nm/s high permeability
- Papp 50-150 Medium
- Papp < 50 nm/sec Low
- BA/AB < 2 no significant efflux
- BA/AB 2-5 modest
- BA/AB > 5 Extensive efflux
- Can use Cyclosporin or Verapmil as PGP inhibitor to confirm PGP substrates.
Incubate with Lucifer yellow at the start to check integrity of membrane Papp should be < 10 nm/s. Always include an internal control, the assay can very from week to week.
In theory you should get 100% recovery from these sort of experiments, reduced recovery can mean binding to the plate of cell mono layer, or it can be due to poor solubility.
Bioavailability
Bioavailability (BA or F) is the fraction of an administered dose of unchanged drug that reaches the systemic circulation. When a drug is administered intravenously, its bioavailability is 100%, however, when a medication is administered via other routes (such as orally), its bioavailability generally decreases, this can be due to a variety of reasons, incomplete absorption, first-pass metabolism, non hepatic metabolism. It is usually designated F and measured in %. The absolute bioavailability F is the dose-corrected area under curve (AUC) non-intravenous divided by AUC intravenous.
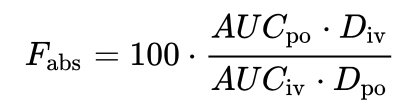
The bioavailability of a drug, after oral administration, is usually less than one (i.e., F< 100%). Various factors reduce the availability of drugs prior to their entry into the systemic circulation, these can include Physical properties of the drug LogP, pKa, HBD/A, solubility the influence of these properties lead to the formulation of the Rule of Five, the drug formulation (immediate release, excipients used, manufacturing methods, modified release – delayed release, extended release, sustained release, etc.). Whether the drug was administered in a fed or fasted state, transporters, metabolism. In addition other co-administered drugs may also have an impact via Cytochrome P450 interactions
Updated 6 December 2019