Bioisosteric Replacements
Aromatic Bioisosteres
Aromatic rings, particularly electron-rich aromatic rings are often prime sites for CYP mediated oxidative metabolism, this can in some cases result in reactive epoxide or quinone intermediates.
Simple replacements
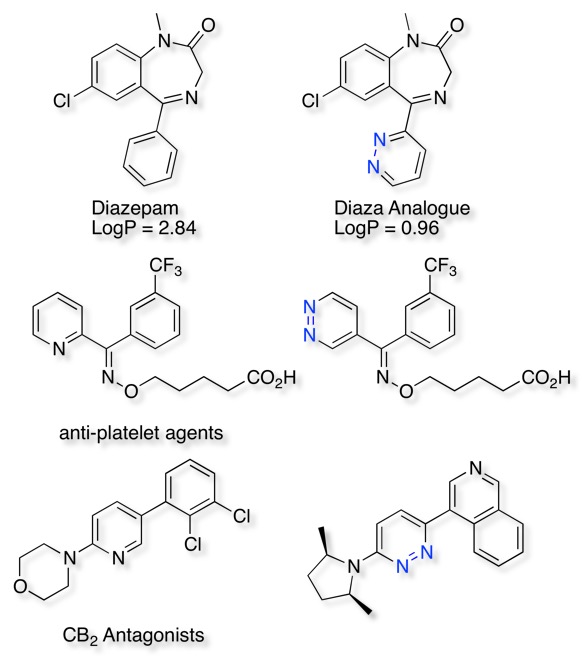
Phenyl, Pyridyl, Pyridazine, Pyrimidine. Introduction of a nitrogen into the ring increases the polarity of the ring and reduces the electron density in the ring. These effects combine to reduce CYP mediated metabolism, however it should be noted that unhindered aromatic nitrogens can be CYP inhibitors. Introduction of heteroatoms also improves water solubility and can impact plasma protein binding.
A variety of 5-membered heteroaromatic rings have been used as phenyl bioisosteres including Thiophene, furan, thiazoles and pyrazoles.
A detailed summary of a matched pair analysis of phenyl ring replacements has been published Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design, DOI.
Often a blocking site of metabolism can be achieved with a simple 4-Fluorophenyl.
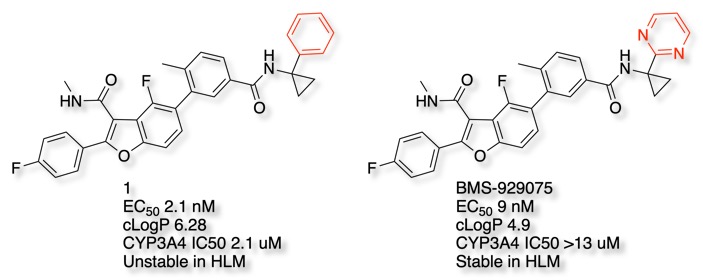
Whilst the hepatitis C virus (HCV) NS5B replicase inhibitor 1 showed excellent affinity it had poor metabolic stability, replacement of the phenyl ring by pyridyl improved the LogP but resulted in potent CYP inhibitors. In contrast, introduction of two nitrogens improved metabolic stability and reduced CYP inhibition, leading to the clinical candidate BMS-929075 DOI.
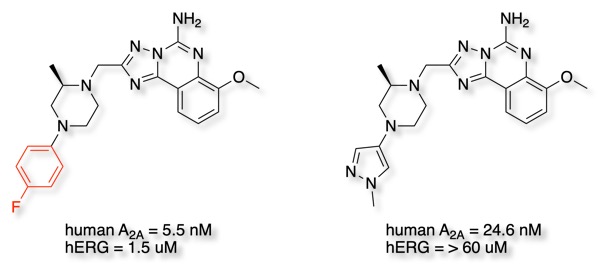
The reduction in lipophilicity of many of the phenyl bioisosteres can also influence hERG inhibition. The adenosine A2A receptor antagonist below was found to be a potent hERG antagonist. Superposition of the lead compound onto MK-499, a benchmark hERG inhibitor, combined with pKa calculations and measurement, identified terminal fluorobenzene to be responsible for hERG activity DOI, whilst replacement of the phenyl by pyridyl served to reduce hERG activity the pyrazole and thiazole were found to eliminate hERG activity.
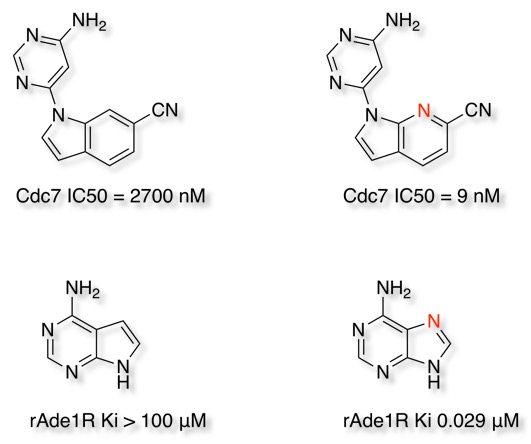
Substitution of a CH group with a N atom in aromatic and heteroaromatic ring systems is a common bioisosteric transformation. Cdc7 kinase is responsible for the initiation and regulation of DNA replication and is a target for cancer therapy. An indole based class of Cdc7 inhibitors were identified. Introduction of a nitrogen at the 7-position dramatically improved affinity suggested to be due to conformational preferences DOI, they also report that both the 7- and 5-aza analogues show improved metabolic stability.
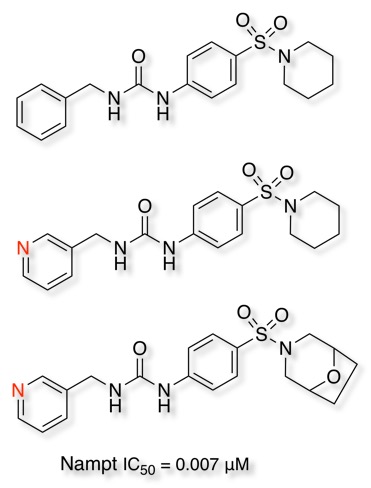
Comparison of Adenine and Deazaadenine Derivatives as Ligands for Adenine Receptors DOI demonstrates a significant reduction in affinity for the deaza compound. Improved metabolic stability for aza analogues has also been reported for a series of inhibitors of nicotinamide phosphoribosyl- transferase (Nampt) DOI, introduction of nitrogen into the benzyl ring affording a 160-fold improvement in metabolic stability, replacement of the piperidine with the 8-oxa-3-azabicyclo[3.2.1]octane gave a further improvement, this compound also showed excellent in vivo antitumor efficacy when dosed orally in an A2780 ovarian tumor xenograft model (TGI of 97% was observed on day 17).
A recent publication by Wermuth DOI describes the influence that introduction of a pyridazine can have on the properties of a molecule. When used as a bioisosteric replacement for a phenyl ring the resulting LogP is reduced by two log units, in other examples they improve water solubility of crystallinity of salts.
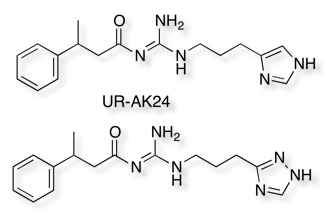
In a very comprehensive study Buschauer et al DOI looked at a wide variety of 5- and 6-membered heterocycles as possible bioisosteric replacements for the imidazole of the histamine H2 agonist UR-AK24. They evaluated all ligands at hH1R, hH2R, hH3R and hH4R in the steady-state GTPase assay, they concluded that in general the replacement of the 1H-imidazol-4-yl ring with isomers or other heterocycles resulted in considerably reduced potency and efficacy. Interestingly the 1H-1,2,4-triazol-3-yl ring provided compounds exhibiting partial to full agonist activity at the hH2R, with little activity at other receptor subtypes. It was also suggested to be relatively stable to enzymic degradation but no data was presented.
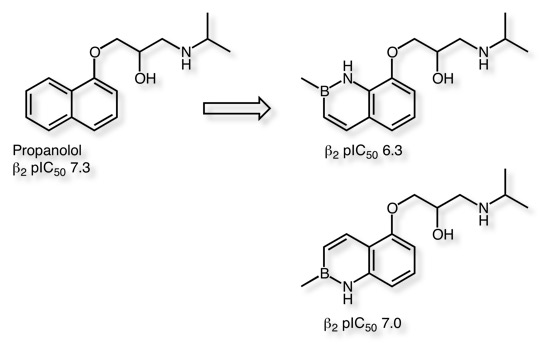
Two benzazaborinine analogues of propranolol have been synthesized and extensively profiled in vitro and in vivo DOI. They showed that the Benzazaborinine analogues showed excellent bioavailability and brain penetration following subcutaneous administration in a pharmacokinetic study in rats.
Phenols are prone to glucuronidation.
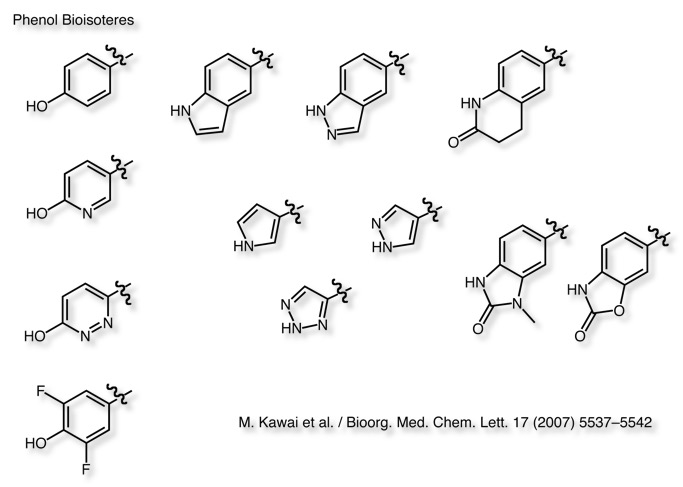
The CF2H group has been proposed as a bioisosteric replacement for the phenol group, it can act as a similar hydrogen bond donor, as confirmed by crystallographic, spectroscopic, and computational methods DOI.
Non-aromatic replacements
A conservative replacement for a phenyl ring would be the corresponding cyclohexyl ring, this is presumably restricted to instances where the phenyl ring is involved in simple hydrophobic interactions and not in any pi-stacking interactions.
In the search for BACE inhibitors DOI the fragment hit (below) was converted to the amide with a benzyl substituent (72uM), replacement of the benzyl ring with the corresponding cyclohexyl afforded a 10-fold increase in affinity.
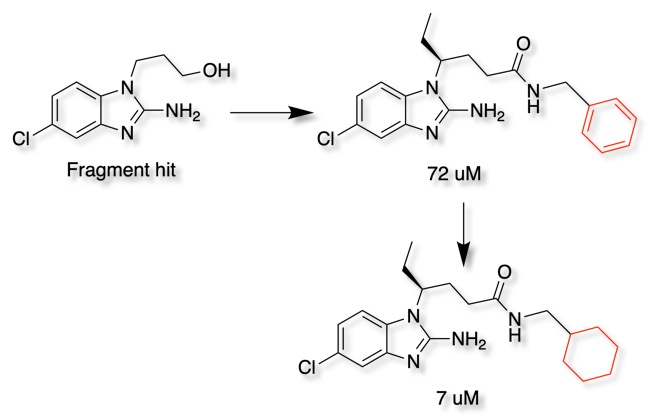
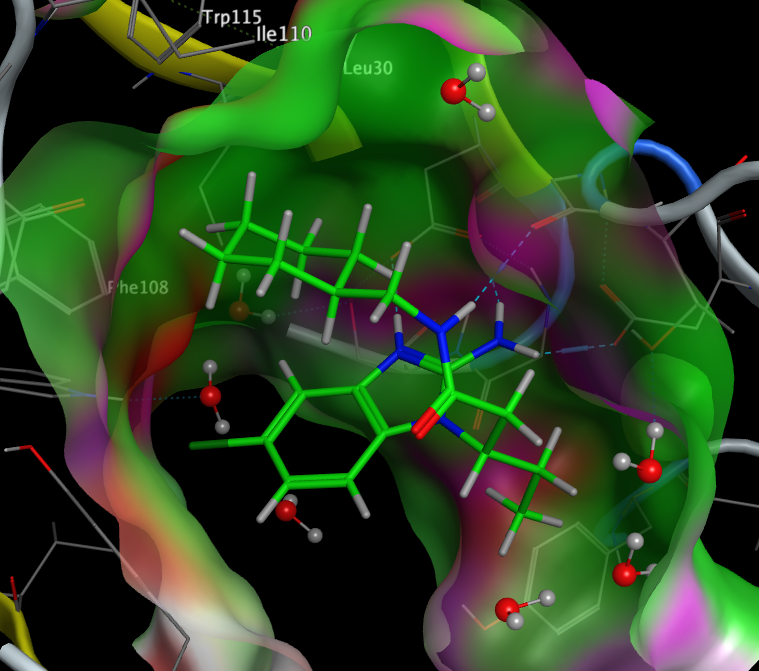
Examination of the crystal structure (PDB ID 3msl) shows the cyclohexyl occupies a hydrophobic pocket lined by Leu30, Ile110, Trp115 and Phe108.
Teddy Zartler drew my attention to my attention to recent publication describing the use of the Bicyclo[1.1.1]pentane Motif as a Nonclassical Phenyl Ring Bioisostere DOI. In this paper they took BMS-708163 (Avagacestat) and replaced one of the phenyl rings with the bicyclo[1.1.1]pentane motif as shown below.
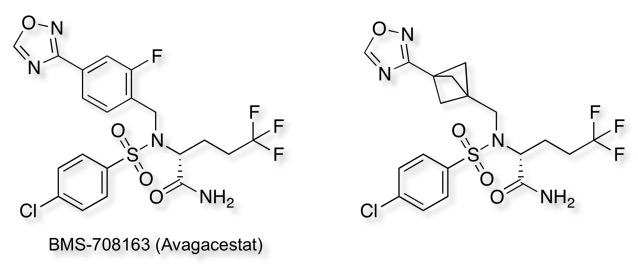
Both compounds have similar γ-secretase inhibition and selectivity suggesting the bicyclo[1.1.1]pentane preserves geometric constraints of the phenyl ring, however it would not be expected to have the same electrostatics or be capable of pi-stacking. Interestingly introduction of the bicyclo[1.1.1]pentane resulted in a significant increase in the aqueous solubility from 0.6 uM to 216 uM. In addition this bioisostere exhibited a greater resistance toward metabolic turnover in cryo-preserved human hepatocytes, and a considerable increase in intrinsic permeability in the RRCK assay.
These sort of bicyclic systems have now been used on multiple programmes and apparently appear in >3000 patents DOI. There have been several alternatives explored in an effort to optimise the bioisostere.
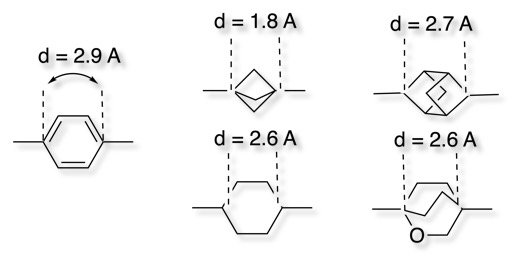
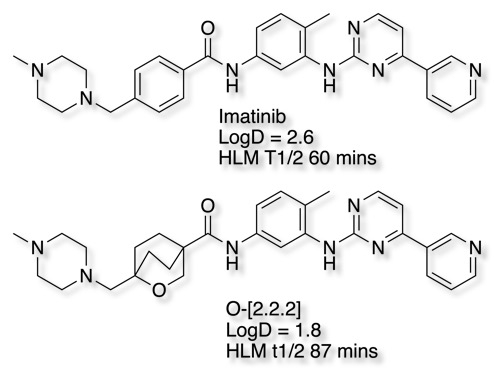
The 2-oxabicyclo[2.2.2]octane is interesting because the presence of the oxygen reduces the lipophilicity. The bioisosteric replacement in Imatinib reduces LogD and increases stability in human liver microsomes DOI.
Worth reading
The Necessary Nitrogen Atom: A Versatile High-Impact Design Element for Multiparameter Optimization. DOI.
Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design, DOI
Last Update 28 Jan 2023