Macrocycles
The publication by Chris Lipinski (Advanced Drug Delivery Reviews 46 (2001) 3 – 26) highlighted the importance of physicochemical properties in drug design leading to the “Rule of Five”. This states “In the discovery setting ‘the rule of 5’ predicts that poor absorption or permeation is more likely when there are more than 5 H-bond donors, 10 H-bond acceptors, the molecular weight (MWT) is greater than 500 and the calculated Log P (CLogP) is greater than 5.” Whilst this has been useful for reducing molecular obesity it is important to note that the original publication excluded certain therapeutic categories that contained many orally active drugs that fell outside the "rule of 5". These excluded categories include antibiotics, antifungals, vitamins and cardiac glycosides, many of these contain macrocyclic orally active drugs.
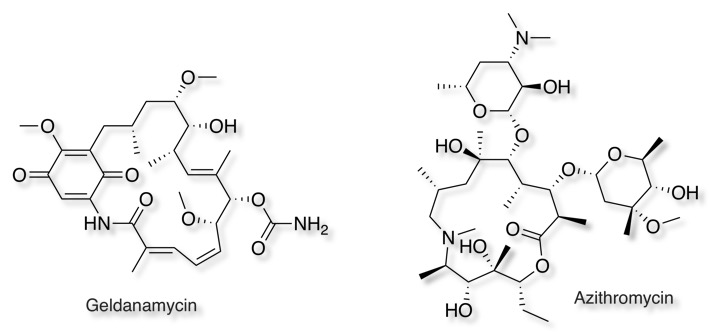
Many of the original macrocycles were developed from natural products and include peptide, polyketides, polyene and combinations, but more recently there has been increased interest in developing novel synthetic macrocycles that have the physical and chemical properties that are needed for cell membrane penetration and oral administration.
Preorganisation
Macrocyclic structure afford a degree of conformational preorganization due to restricted rotation and conformational restraint, if the macrocycle can be locked in the active binding conformation this can yield substantial increases in binding affinity.
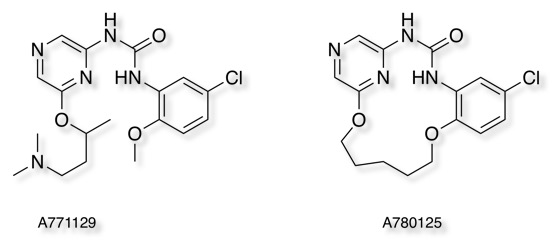
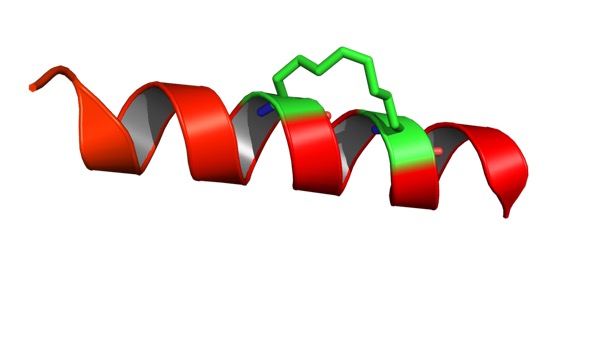
In a study of Checkpoint Kinase 1 Inhibitors DOI the authors compare an acyclic ligand A771129 (green, PDB 2E9P) and the corresponding macrocycle A780125 (purple, PDB 2E9U), the macrocycle displays a 2-fold improvement in affinity despite the loss of an interaction between the tertiary amine and Glu91.
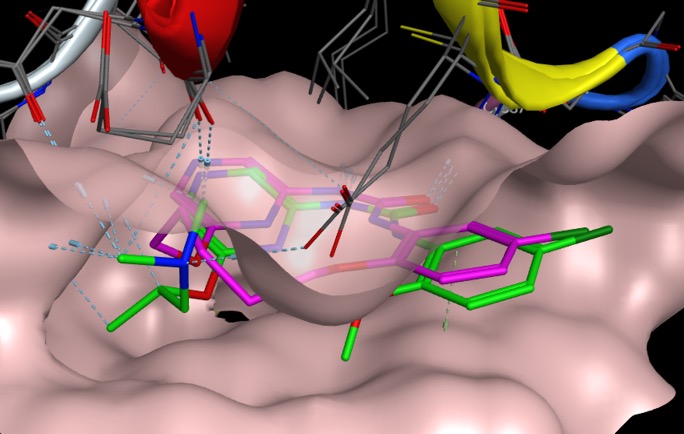
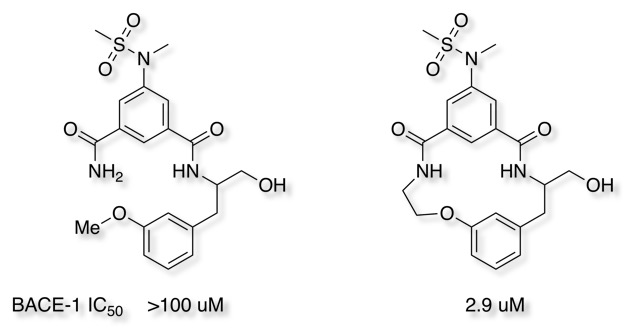
Macrocyclization of an acyclic BACE1 inhibitor dramatically improved potency, the authors also comment that "Macrocyclization resulted in significantly improved physical properties when compared to the initial lead structure".
Macrocyclic Peptides
Despite their interesting and versatile biological activities, and potential as drugs, peptides are generally not cell permeable and have unreliable pharmacokinetics, with low bioavailability, high renal clearance and significant proteolytic degradation. In an effort to improve these properties a variety of strategies have been adopted, building macrocyclic peptides using peptidic or disulphide bonds to build the macrocycle, introduction of unnatural amino acids, and the using non-peptide motifs to construct the macrocycle.
Cyclotides are ultra-stable peptides derived from plants. They are around 30 amino acids in size and are characterised by their head-to-tail cyclic backbone and the interlocking arrangement of three disulfide bonds. They have a well-defined three-dimensional structure and have been used as a scaffold to design novel peptides PubMed.
A stapled peptide is a peptide that has a synthetic brace ("staple") created by covalently linking the side-chains of two amino acids, thereby forming a peptide macrocycle. This stabilises the conformation of the peptide and may confer metabolic stability. . Screening a library of stabilized Alpha-Helix of BCL-2 domains (SAHBs) identified MCL-1 BH3 helix is itself a potent and exclusive MCL-1 inhibitor DOI.
Bicycle Therapeutics have taken this one step further and developed bridged bicyclic peptides DOI in which conjugation with tris-(bromomethyl)benzene via the reactive cysteines generated repertoires of peptide conjugates with two peptide loops anchored to a mesitylene core.
Physicochemical Properties and Pharmacokinetics
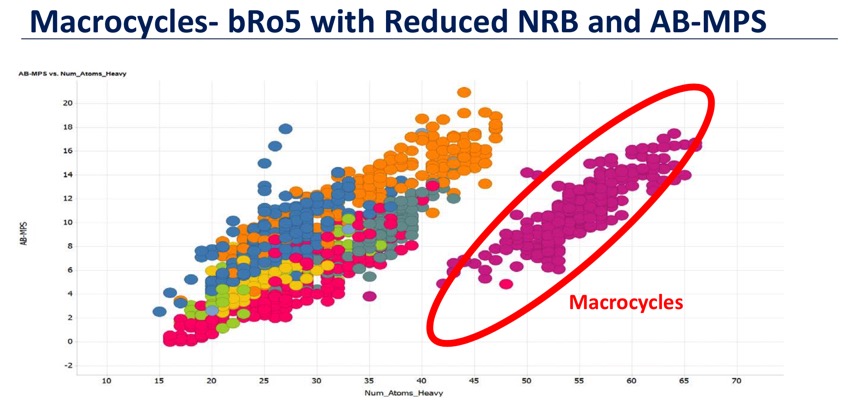
Whilst the rule of 5 (Ro5) has provided a useful way to describe small molecule drug space it is also clear that there are a significant number of molecular classes that exist beyond the rule of 5 boundaries (bRo5). In a review of the AbbVie compound collection DOI they were able to identify key findings that might explain the success (or failure) of bRo5 projects. From an analysis of a variety of calculated physicochemical properties they proposed a simple multiparametric scoring function (AB-MPS) was devised that correlated preclinical PK results with cLogD, number of rotatable bonds, and number of aromatic rings.
AB-MPS = Abs(cLogD-3) + NAR + NRB
Where cLogD was the calculated LogD (ChemAxon), NAR is number of aromatic rings, NRB is number of rotatable bonds, with AB-MPS values of ≤14 predicting a higher probability of success. Compounds with AB-MPS <14 on average had better bioavailability and PAMPA cell penetration. Whilst this analysis did not focus on macrocycles a subsequent analysis of Macrocycles showed that despite high molecular weights they had good AB-MPS scores (presented by Phil Cox at Design rules for macrocycles http://www.drugdiscoverychemistry.com/Macrocyclics/). They also showed a correlation between AB-MPS score and cell penetration using published data from DOI. Macrocycles occupy a special area of property space bRo5 due to a much more reduced number of rotatable bonds because of the constraints imposed by the cyclic structure. A Vortex script to calculate AB-MPS is available.
Macrocycles are usually high molecular weight and have a large calculated polar surface area (PSA), PSA is defined as a sum of surfaces of polar atoms (usually oxygens, nitrogens and attached hydrogens) in a molecule, (PSA has been shown to correlate very well with the human intestinal absorption, Caco-2 monolayers permeability, and blood-brain barrier penetration) Thus calculated PSA is independent of the conformation of the molecule and macrocycles can adopt different conformation in various media, hiding polar atoms or forming intramolecular hydrogen bonds, thus retaining good cell permeability. This was elegantly demonstrated by Scott Lokey et al DOI who looked at a series of cyclic and acyclic peptides and showed that membrane diffusion rates differed by nearly two log units. The most permeable diastereomer, cyclo[d-Leu-d-Leu-Leu-d-Leu-Pro-Tyr], exhibited a passive membrane diffusion rate comparable to that of the orally available drug cyclosporine A. In addition oral bioavailability can be obtained at molecular weights up to and above 1 kDa and polar surface areas ranging toward 250 Å2 DOI.
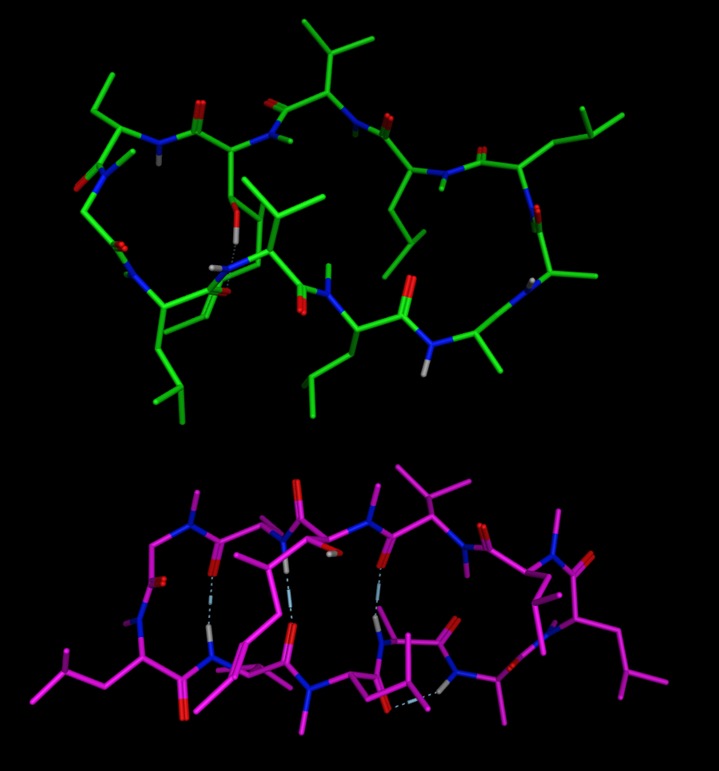
The crystal structure of cyclosporin A (CSD DEKSAN) shown in purple below shows a “closed” twisted β-pleated sheet conformation with four intramolecular hydrogen bonds, however when binding to cyclophilin, PDB code 2RMA, cyclosporin A adopts an “open” conformation without intramolecular hydrogen bonds (shown in green below). Similar observations have been made following molecular dynamics simulations of Cyclosporin A in Polar and Apolar Environments DOI.
Biological Targets Addressed by Macrocycles
Whilst initially most of the macrocyclic molecules isolated from natural sources were anti-infective agents, macrocycles have now been used in a wide range of therapeutic targets.
Oncology
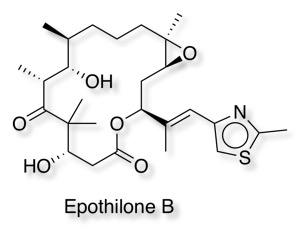
Epothilone B, an approved drug for the treatment of metastatic breast cancer, has been shown to bind at the interface of two tubulin subunits.
Immunosuppression
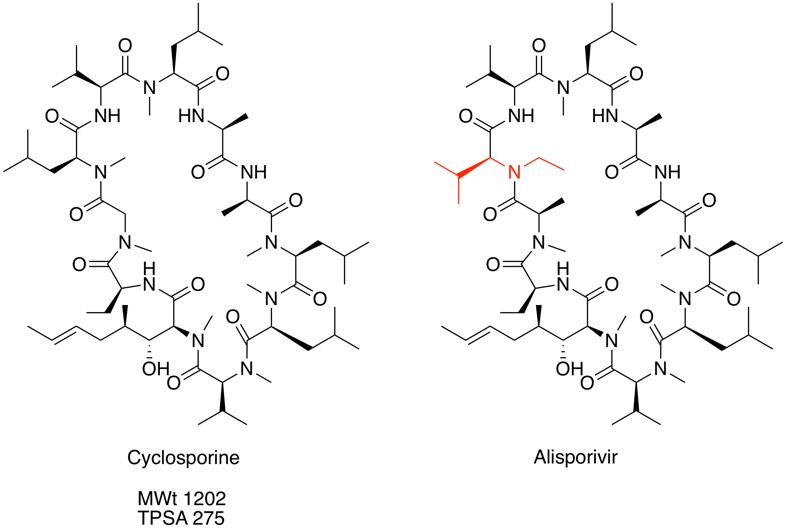
Cyclosporine is an immunosuppressant that acts by forming a complex with cyclophilin to block the phosphatase activity of calcineurin, which in turn decreases the production of inflammatory cytokines by T‐lymphocytes. Despite a molecular weight of 1202 and TPSA 275 Cyclosporin has been shown to be orally bioavailable in man (20-60%) DOI.
Hepatitis C
Interestingly, cyclosporine A was shown to inhibit different virus replication (HIV, HCV, HBV) using phenotypic screening and a single N-methyl-Leucine to N-ethyl-Valine amino-acid replacement (shown in red above) delivered a compound with a totally different profile. Debio-025 (Alisporivir) is ten-fold more potent as a HCV inhibitor in the replicon cell-based assay versus Cyclosporine and lacks the immunosuppressive activity. Alisporivir blocks HCV replication by neutralizing the peptidyl-prolyl isomerase activity of the abundant host cytosolic protein, cyclophilin A.
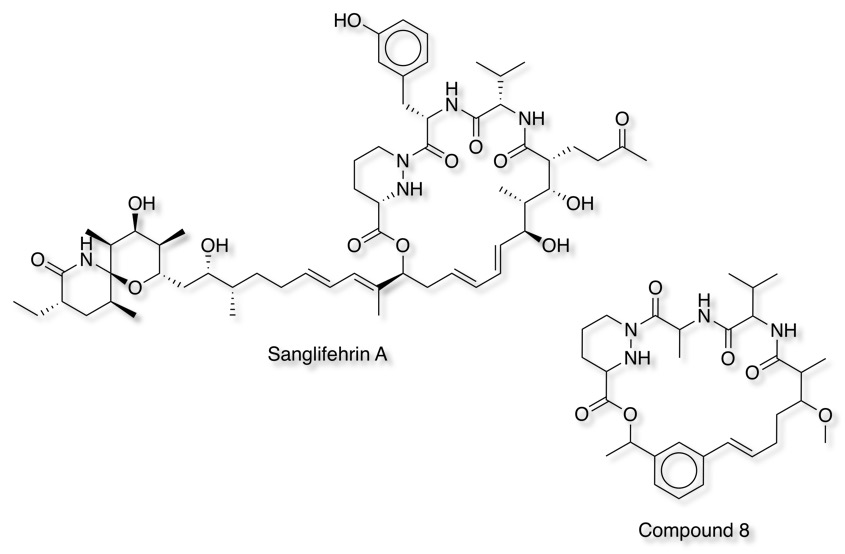
Sanglifehrin A (SFA) is a novel immunosuppressive natural product that also binds to cyclophilin but is structurally distinct from cyclosporin A. Degradation studies on sanglifehrin A determined that removal of the C24 polyketide side chain eliminated the immunosuppressive properties of the molecule but retained the binding to cyclophilins. DOI. Replacing the C18–C21 diene unit of sanglifehrin with a styryl group led to compound 8 that displayed a novel binding mode in which the styrene moiety engaged in a π-stacking interaction with Arg55 of cyclophilin A.
HCV protease
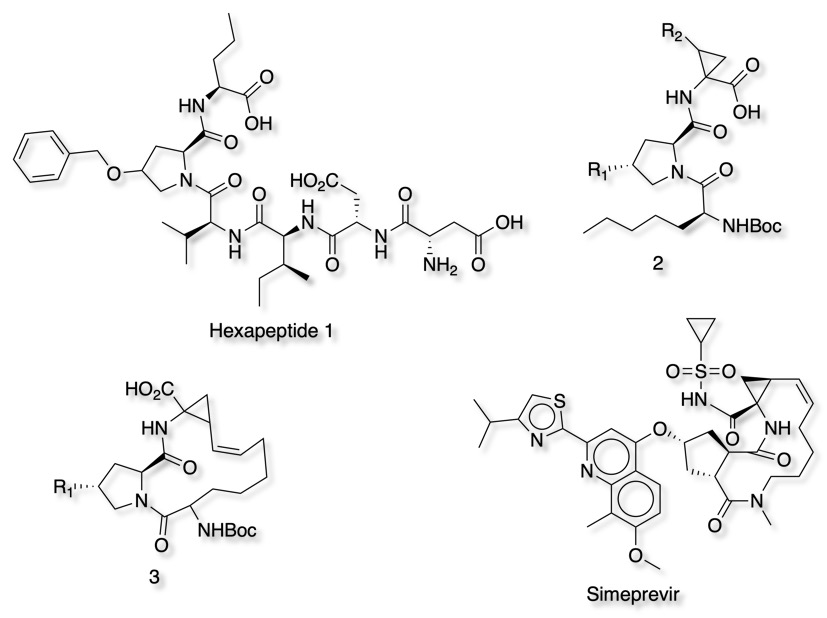
Nonstructural protein 3 (NS3) A3EZJ3 , also known as p-70, is a viral nonstructural protein that is 70 kDa cleavage product of the hepatitis C virus polyprotein. It is a serine protease for which there are a number of launched drugs. Much of this work was based on the observation that N-terminal peptide products derived from substrates are competitive inhibitors of the enzyme. Hexapeptide 1 was used to explore conformational flexibility and the interactions with the binding site. Truncation and conformational restrictions lead to inhibitors of type 2. Subsequent macrocyclisation 3 lead to a significant increase in potency, further modification yielded Simeprevir
Mouse Controls
| Movement | Mouse Input | Touch Input | ||
|---|---|---|---|---|
| Rotation | Primary Mouse Button | Single touch | ||
| Translation | Middle Mouse Button or Ctrl+Primary | Triple touch | ||
| Zoom | Scroll Wheel or Second Mouse Button or Shift+Primary | Pinch (double touch) | ||
| Slab | Ctrl+Second | Not Available |
Worth Reading
Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery DOI.
Macrocycles in new drug discovery DOI.
Structure-based macrocycle design in small-molecule drug discovery and simple metrics to identify opportunities for macrocyclization of small-molecule ligands DOI
Updated 5 May 2019