Hepatotoxicity
After oral dosing the liver is the first major organ to be exposed to high doses of the drug and is thus a major target organ in the repeated dose nonclinical safety studies used to support clinical trials.
In Vivo Studies
High dose studies in safety species are undertaken to identify potential toxicities and to determine safety margins, Clinically, the most relevant reactions include liver necrosis, hepatitis, cholestasis, vascular changes and steatosis. A drug can cause liver toxicity via multiple mechanisms, it can be the result of a direct action of the parent compound or indirectly through reactive metabolites. The drug or its metabolites may cause liver toxicity after specific receptor binding, or reactive metabolites can react with hepatic macromolecules, all leading to direct cytotoxicity. In addition, Immune-mediated idiosyncratic drug reaction has been responsible for numerous serious hepatotoxic events in humans and is described in a separate section.
Of particular note are a range of serum markers that can give an early signal of potential hepatotoxicity, in particular, increases in the levels of the liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum, in combination with increased bilirubin levels. Detailed plasma chemistry evaluation can then provide further diagnostic information.
ALT is considered a more specific and sensitive indicator of hepatocellular injury than AST. An increase of ALT activity in the range of 2-4 fold and higher compared to control would be considered a concern.
The tables below are intended to provide an indication of the levels of serum markers in untreated rat and are taken from a survey of serum chemistry parameters taken from rat blood samples from 98 different labs (J Toxicol Sci. 1997 Feb;22(1):25-44).
| Parameter (Rat) | Units | Mean | Typical Range |
|---|---|---|---|
| Phosphorous | g/dL | 5.52 | 5.5-10.4 |
| Albumin | g/dL | 4.21 | 3.4-4.8 |
| Glucose | mg/dL | 131 | 70-208 |
| ALT | U/L | 44 | 18-48 |
| AST | U/L | 106 | 65-203 |
| ALP | U/L | 190 | 62-230 |
| Bilirubin | mg/dL | 0.06 | 0.05-0.15 |
| Creatine | mg/dL | 0.27 | 0.2-0.6 |
During hepatocellular necrosis ALT is elevated as is sorbitol dehydrogenase (SDH). Liver specific enzymes that indicate intrahepatic ductular cholestasis include alkaline phosphatase (ALP). Gamma glutamyltransferase (GGT) is specific for cholestasis and is commonly used to assess cholestasis in non-rodents. ALP is more sensitive but much less specific than GGT.
ALT levels in normal mice appear to vary significantly in the literature, and the levels have been shown to vary depending on how the mice were handled, The Effect of Handling Techniques on Serum ALT Activity in Mice (JOURNAL OF APPLIED TOXICOLOGY, VOL. 5, NO. 3,1985).
There is less publicly available data for dogs but the table below gives some that I have found in the literature DOI
| Parameter(Dog) | Units | Mean |
|---|---|---|
| Phosphorous | g/dL | 6.0 |
| Albumin | g/dL | 3.2 |
| Glucose | mg/dL | 131 |
| ALT | U/L | 28 |
| AST | U/L | 34 |
| ALP | U/L | 280 |
| Bilirubin | mg/dL | 0.07 |
| Creatine | mg/dL | 0.79 |
In Vitro Studies
Whilst in vitro systems offer significant advantages in speed and capacity there is always the concern that an in vitro system may not reproduce all the metabolic events seen in vivo. Liver slices, cell lines, and primary hepatocytes have all been evaluated as models in in vitro liver toxicity testing.
Subcellular fractions (S-9, microsomes, mitochondria)
High availability, can be used in high-throughput screenings.Used for mitochondrial dysfunction/energy metabolism studies Provide some of the drug-metabolising capacity of cellular systems, but would need to combine/test multiple fractions to get complete picture
Little literature to support use in toxicity studies, No preservation of cell structures and functions, extremely simplified models, lacking whole gene regulation systems, cell- cell interactions, bile canaliculi, transporters, cell structures and function
Liver-derived cell lines
Human cell lines include HepG2, Hep3B, HepaRG, HepZ, C3A,THLE; or non-human hepatic cell lines: HTC, BRL3A and NRL clone 9, Fa32 and WIF-B9
Unlimited availability, easy to use and reasonably high-throughput. Possibility of genetic manipulation (single enzyme/ transporter studies) - High reproducibility
Lack/low expression of key hepatic functions, Limited/partial drug-metabolism due to lower levels of P450 enzymes
Primary cultured hepatocytes
Easy to use for Viability and functional studies. Potential use for longer-term studies. Possible donor variability.
Isolated hepatocytes
Highly available (cryopreservation), Easy handling (reasonably high-throughput). Viability and functional studies (enzymes, transporters) Short-term viability (2-4 hr), No cell-cell contacts or cell-matrix interactions
Liver slices
Preserved Structure and enzymes, Cellular heterogeneity (includes non-parenchymal cells) - Histologic examination is possible. Functional assessment (enzymes, transporters) Limited viability and function Scarce availability (requires fresh liver) - High intra-assay variability. Technically demanding. Viable for several days.
Immune-based hepatotoxicity
Signals for an immune-based liver damage may be obtained from histopathological analysis (e.g. an increase in parenchymal eosinophils, mild hepatic inflammation or granulomas). It should be noted that presently there are no validated models to quantify the potential liability or risk involved in immune-mediated hepatotoxicity testing. See also Idiosyncratic toxicity.
Some molecular mechanisms of Hepatotoxicity
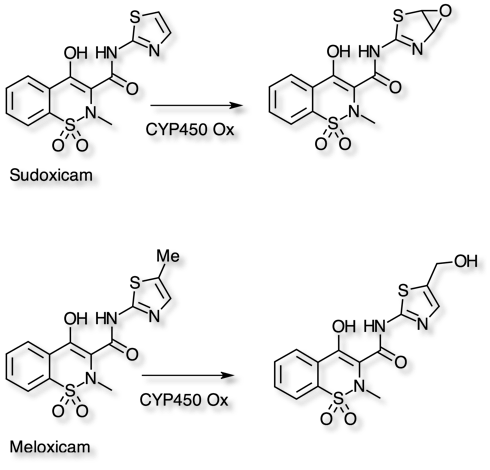
Metabolism of xenobiotics to yield reactive intermediates has been shown to result in hepatotoxicity. Sudoxicam and meloxicam are nonsteroidal anti-inflammatory drugs (NSAIDs) that only differ by a single methyl group. Interestingly, sudoxicam has been associated with several cases of severe hepatotoxicity that led to its discontinuation, while meloxicam has been in the market for over a decade and is devoid of hepatotoxicity. In vitro investigation Chem. Res. Toxicol. 2008, 21, 1890–1899 in human liver microsomes demonstrated differing routes of metabolism. For Sudoxicam oxidation of the thiazole ring affords a range of potential reactive metabolites, in contrast for Meloxicam hydroxylation of the thiazole methyl groups affords a benign route for metabolism.
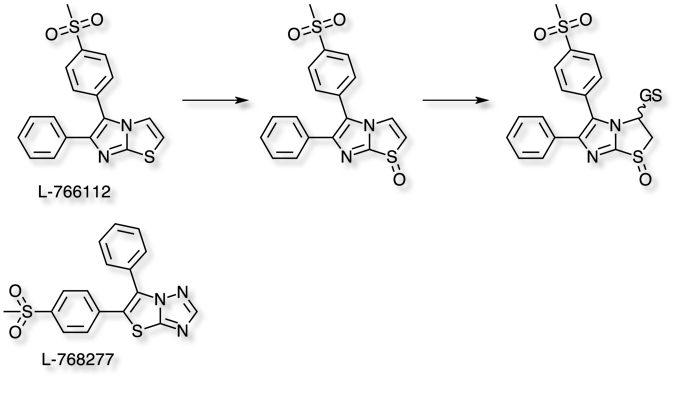
Oxidative metabolism of the imidazo[2,1-b]thiazole ring of the COX-2 inhibitor L-766112 has also been reported DOI to lead to a reactive intermediate, a pathway that is not observed for the related analogue L-768277.
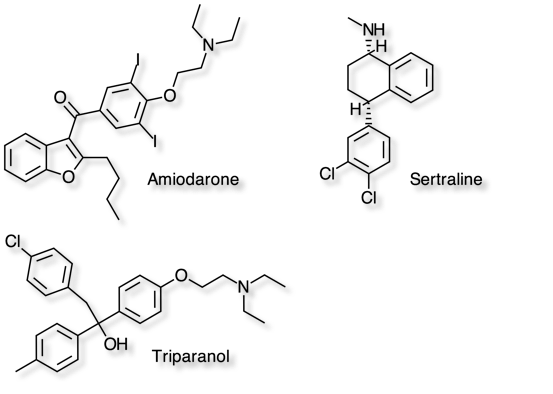
Cationic amphiphilic drugs such as Amiodarone, Sertaline and Triparanol have been identified as inducers of phospholipidosis, a dose-dependent process in which the drug accumulates within lysosomes. Many of these drugs have been shown to interfere with lysosomal phospholipase.
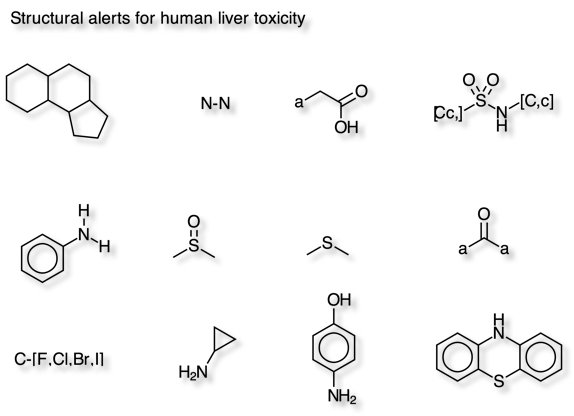
Liver toxicity Filter
Wallqvist et al DOI have identified 12 molecular fragments that are strongly associated with drug-induced human liver injuries using data from LiverTox a database of information on the diagnosis, cause, frequency, patterns, and management of liver injury attributable to prescription and nonprescription medications, herbals and dietary supplements.
There is a Vortex script for flagging compounds with a potential hepatotoxicity liability here.
It would be useful to be able to predict ahead of synthesis whether a molecule was likely to cause liver injury and that is the function of DILIpredictor DOI. Using data form several thousand molecules and a variety of different assays (both in vitro and in vivo) and different species the authors have developed a predictive model. The attraction of this approach is in addition to giving an early flag of potential DILI it also highlights potential species differences and can give an insight into the mechanism.
DILIPredictor required only chemical structures as input for prediction and is publicly available at https://broad.io/DILIPredictor for use via web interface (please don't submit confidential molecules) and with all code available for download from GitHub
https://github.com/srijitseal/DILI_Predictor
I installed like this since it currently does not run with the latest version of python
conda create -n DILIpred python=3.10
conda activate DILIpred
pip install DILIpred
It can then beinstalled as follows.
conda create -n DILIpred python=3.10
conda activate DILIpred
pip install DILIpred
It can then be usef as follows
(DILIpred) chrisswain@Mac-Studio ~ % dilipred -smiles "C[C@@H]1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C"
If you use DILIPred in your work, please cite: Improved Detection of Drug-Induced Liver Injury by Integrating Predicted In Vivo and In Vitro Data Srijit Seal, Dominic Williams, Layla Hosseini-Gerami, Manas Mahale, Anne E. Carpenter, Ola Spjuth, and Andreas Bender doi: https://doi.org/10.1021/acs.chemrestox.4c00015
100%███████████████████████████████████████████████████████████████ 1/1 [00:01<00:00, 1.14s/it] 2024-07-12 08:29:44.777 | CRITICAL | dilipred.main:predict:458 - The compound is predicted DILI-Positive
The detailed output is contained in a file created.
source,assaytype,description,value,pred,SHAP contribution to Toxicity,SHAP,smiles,smilesr DILI,DILIstFDA,This is the predicted FDA DILIst label,0.8187885151383966,1,N/A,N/A,C[C@@H]1C2=NN=C(N2CCN1C(=O)C3=CC=C(C= C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O)c2ccc(F)cc2)C3C)n1 Diverse DILI C,Heterogenous Data ,"Transient liver function abnormalities, adverse hepatic effects",0.7393781727510759,True,Positive,0.003926801602711443,C[C@@H] 1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O )c2ccc(F)cc2)C3C)n1 BESP,Mechanisms of Liver Toxicity,BESP Bile Salt Export Pump Inhibition,0.5655727513227511,True,Positive,0.0003070244326849143,C[C@@H ]1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(= O)c2ccc(F)cc2)C3C)n1 Mitotox,Mechanisms of Liver Toxicity,Mitotox ,0.10973983865879627,False,Positive,0.0006130947805277731,C[C@@H]1C2=NN= C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O)c2ccc( F)cc2)C3C)n1 Reactive Metabolite,Mechanisms of Liver Toxicity,Reactive Metabolite Formation,0.19967540492325553,False,Negative,-0.001048102741727997,C[C@@ H]1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C( =O)c2ccc(F)cc2)C3C)n1 Human hepatotoxicity,Human hepatotoxicity,"Human hepatotoxicity, hepatobiallry",0.7196576912119554,True,Positive,0.007802364907899422,C[C @@H]1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN( C(=O)c2ccc(F)cc2)C3C)n1 Animal hepatotoxicity A,Animal hepatotoxicity,"Rat, chronic oral administration, Hepatic histopathologic effects, ToxRefDB",0.5867747455286331,True,Positive,0.0030731971130978854,C[C@@H] 1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O )c2ccc(F)cc2)C3C)n1 Animal hepatotoxicity B,Animal hepatotoxicity,"Hepatocellular hypertrophy, rats, ORAD, HESS",0.6646590439473917,True,Positive,0.0013360236463188587,C[C@@H]1C2= NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O) c2ccc(F)cc2)C3C)n1 Preclinical hepatotoxicity,Animal hepatotoxicity,"Preclinical hepatotoxicity data from PharmaPendium, Leadscopre, and internal repository with 14- to 28-day rat study data",0.8576928962241468,True,Positive,0.011692057666492625,C[C@@H]1C2= NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O) c2ccc(F)cc2)C3C)n1 Diverse DILI A,Heterogenous Data ,Large-scale and diverse ddrug induced liver injury dataset,0.6324274304660036,True,Positive,0.003398315762493277,C[C@@H]1C2 =NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O) c2ccc(F)cc2)C3C)n1 source,assaytype,description,value,pred,SHAP contribution to Toxicity,SHAP,smiles,smilesr DILI,DILIstFDA,This is the predicted FDA DILIst label,0.8187885151383966,1,N/A,N/A,C[C@@H]1C2=NN=C(N2CCN1C(=O)C3=CC=C(C= C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O)c2ccc(F)cc2)C3C)n1 Diverse DILI C,Heterogenous Data ,"Transient liver function abnormalities, adverse hepatic effects",0.7393781727510759,True,Positive,0.003926801602711443,C[C@@H] 1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O )c2ccc(F)cc2)C3C)n1 BESP,Mechanisms of Liver Toxicity,BESP Bile Salt Export Pump Inhibition,0.5655727513227511,True,Positive,0.0003070244326849143,C[C@@H ]1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(= O)c2ccc(F)cc2)C3C)n1 Mitotox,Mechanisms of Liver Toxicity,Mitotox ,0.10973983865879627,False,Positive,0.0006130947805277731,C[C@@H]1C2=NN= C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O)c2ccc( F)cc2)C3C)n1 Reactive Metabolite,Mechanisms of Liver Toxicity,Reactive Metabolite Formation,0.19967540492325553,False,Negative,-0.001048102741727997,C[C@@ H]1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C( =O)c2ccc(F)cc2)C3C)n1 Human hepatotoxicity,Human hepatotoxicity,"Human hepatotoxicity, hepatobiallry",0.7196576912119554,True,Positive,0.007802364907899422,C[C @@H]1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN( C(=O)c2ccc(F)cc2)C3C)n1 Animal hepatotoxicity A,Animal hepatotoxicity,"Rat, chronic oral administration, Hepatic histopathologic effects, ToxRefDB",0.5867747455286331,True,Positive,0.0030731971130978854,C[C@@H] 1C2=NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O )c2ccc(F)cc2)C3C)n1 Animal hepatotoxicity B,Animal hepatotoxicity,"Hepatocellular hypertrophy, rats, ORAD, HESS",0.6646590439473917,True,Positive,0.0013360236463188587,C[C@@H]1C2= NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O) c2ccc(F)cc2)C3C)n1 Preclinical hepatotoxicity,Animal hepatotoxicity,"Preclinical hepatotoxicity data from PharmaPendium, Leadscopre, and internal repository with 14- to 28-day rat study data",0.8576928962241468,True,Positive,0.011692057666492625,C[C@@H]1C2= NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O) c2ccc(F)cc2)C3C)n1 Diverse DILI A,Heterogenous Data ,Large-scale and diverse ddrug induced liver injury dataset,0.6324274304660036,True,Positive,0.003398315762493277,C[C@@H]1C2 =NN=C(N2CCN1C(=O)C3=CC=C(C=C3)F)C4=NC(=NS4)C,Cc1nsc(-c2nnc3n2CCN(C(=O) c2ccc(F)cc2)C3C)n1
Overall a useful tool to have to hand.
Worth reading The Identification of Toxicophores for the Prediction of Mutagenicity, Hepatotoxicity and Cardiotoxicity EMEA guildelines on hepatotoxicity
Updated 17 Jul 2024