Mutagenicity
The regulatory guidelines are available online, ICH Harmonised Tripartite Guideline S2(R1). Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use. 2011, link.
Tests to evaluate mutagenic potential
Mutagenicity refers to the induction of permanent transmissible changes in the amount or structure of the genetic material in cells or organisms. These changes may involve a single gene (point mutations), a block of genes or entire chromosomes (structural or numerical chromosome aberrations). In many cases, genotoxicity may lead to cancer. Thus, genotoxicity testing is performed to assess the potential of substances to induce genotoxic effects which may cause heritable damage or lead to cancer in humans.
Ames Test
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in the DNA of the test organism. It is quick and convenient and was first described by Bruce Ames in the 1970s DOI.
The Ames test uses several strains of the bacterium Salmonella typhimurium that carry mutations in genes involved in histidine synthesis. These strains are auxotrophic mutants, i.e. they require histidine for growth, but cannot produce it. The method tests the capability of the tested substance in creating mutations that result in a return to a "prototrophic" state, so that the cells can grow on a histidine-free medium.
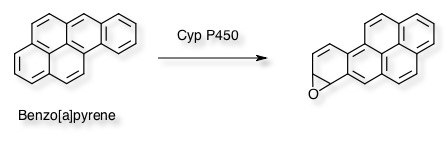
The above experiment only evaluates the mutagenic potential of the parent compound, liver extracts (e.g. human S9 fraction) can be added to help evaluate metabolites. An example of which is Benzo[a]pyrene which is oxidised to a variety of reactive metabolites including epoxides by Cytochrome p450 enzymes.
The results of many AMES tests are now in the public domain and there are several compiled data sets including, Benchmark Data Set for in Silico Prediction of Ames Mutagenicity, J. Chem. Inf. Model., 2009, 49 (9), pp 2077–2081 DOI. This data set describes the structures and experimentally determined AMES activity for over 6500 molecules. These data sets can be used to build predictive computational models.
These models include
- Predicting AMES activity Jupyter Notebook.
- An in Silico Method for Predicting Ames Activities of Primary Aromatic Amines by Calculating the Stabilities of Nitrenium Ions
- The Benigni / Bossa rulebase for mutagenicity and carcinogenicity – a module of Toxtree
The mouse lymphoma assay
The mouse lymphoma TK assay (MLA) is designed to predict risk assessment prior to in vivo testing. The test has the potential to detect mutagenic and clastogenic events at the thymidine kinase (tk) locus of L5178Y mouse lymphoma tk ( +/- ) cells by measuring resistance to the lethal nucleoside analogue triflurothymidine (TFT) DOI
Micronucleus test
The in vitro micronucleus test detects genotoxic damage in interphase cells. The in vitro micronucleus test provides an alterative to the chromosome aberration test. DOI.
A micronucleus is formed during the metaphase/anaphase transition of mitosis (cell division). It may arise from a whole lagging chromosome (aneugenic event leading to chromosome loss) or an acentric chromosome fragment detaching from a chromosome after breakage (clastogenic event) which do not integrate in the daughter nuclei.
BlueScreen
BlueScreen™ HC is an accurate and fast in vitro human cell-based assay for the assessment of genotoxicity and cytotoxicity of compounds and mixtures. BlueScreen HC uses the human-derived, p53 competent, TK6 cell line to host a Gaussia luciferase (GLuc) reporter system which exploits the proper regulation of the GADD45a gene. Exposure to a genotoxic compound increases the expression of GADD45a and hence the induction of Gaussia luciferase production in the test strain. DOI.
In vivo Tests
An in vivo test for micronuclei or chromosome aberrations in rodent hematopoietic cells is also required. Both rats and mice are considered appropriate for use in the bone marrow micronucleus test. Micronuclei can also be measured in immature (e.g., polychromatic) erythrocytes in peripheral blood in the mouse, or in the newly formed reticulocytes in rat blood
Updated 27 January 2017