Aggregation
Promiscuous inhibition caused by small molecule aggregation is a major source of false positive results in high-throughput screening. To mitigate this, use of a nonionic detergent such as Triton X-100 or Tween-80 has been studied, which can disrupt aggregates, and is now common in screening campaigns DOI.
Three key results emerge from this study: first, detergent-dependent identification of aggregate-based inhibition is feasible on the large scale. Second, 95% of the actives obtained in this screen are aggregate-based inhibitors. Third, aggregate-based inhibition is correlated with steep dose-response curves, although not absolutely.
Physicochemical Properties of Aggregators
A recent particularly valuable publication, Irwin, Duan, Torosyan, Doak, Ziebart, Sterling, Tumanian and Shoichet, J Med Chem, 2015, 58(1 7), 7076-7087 DOI, has collated over 12,000 organic molecules known to act as aggregators at concentrations used in screening campaigns, and provides a resource Aggregation Advisor that can be used to try and predict possible false positives. However in many instances it would be unwise to submit proprietary information to the public web service. Potential aggregators are flagged based on calculated LogP >3 and/or similarity >0.85 to a known aggregator.
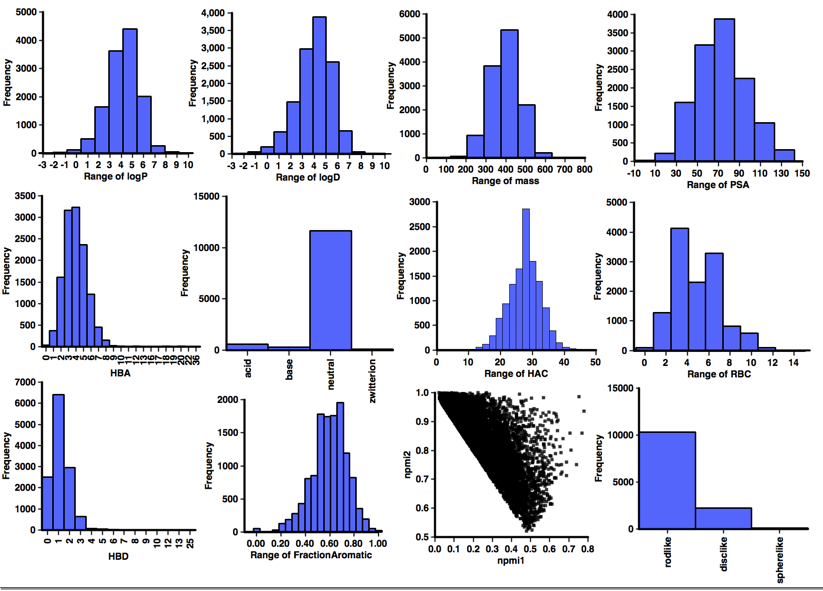
A profile of the physicochemical properties (HBD, HBA, PSA, HAC, LogP, LogD, MWt, RBC) was generated using an Applescript that uses evaluate from ChemAxon to calculate the physicochemical properties and Aabel to construct the histograms. I also used it to determine pKa in order to identify acidic or basic groups and categorized the aggregators accordingly, in addition I calculated the fraction of aromatic atoms (number of aromatic atoms/number of heavy atoms). I’ve also included npri (Normalized ratio of principle moments of inertia) as described by Sauer WH, Schwarz MK (2003) Molecular shape diversity of combinatorial libraries: A prerequisite for broad bioactivity. J Chem Inf Comput Sci 43:987–10030. DOI this was calculated using MOE.
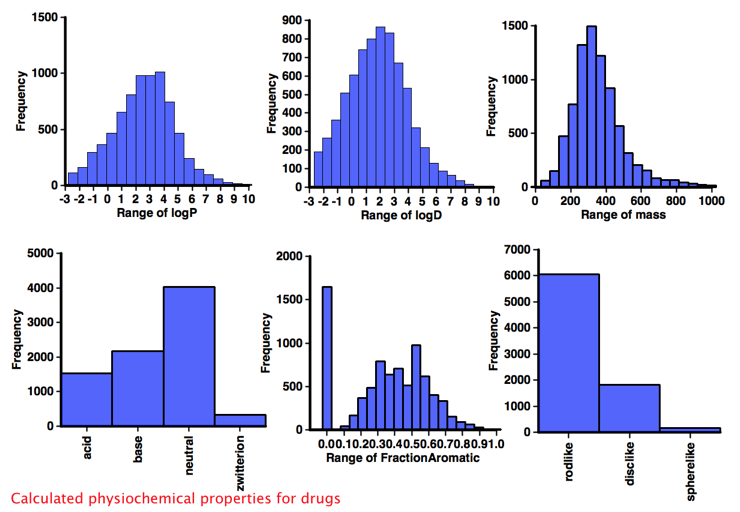
Comparison with Drugs
It would be interesting to compare the calculated physicochemical properties of the known aggregators with "non-aggregators" but unfortunately I could not find a dataset of comparable size, so instead I used "approved drug category" provided by ChEMBL, after selecting only small molecules I calculated the same physicochemical properties and a selection are shown below. One very striking feature of this analysis when compared to the corresponding data for "drugs' shown in the plots below is the lack of ionisable groups observed in the aggregator compounds. Whilst the majority of aggregators do have a calculated LogP >3, however because of the lack of ionisable groups it might be better to use a cut off of LogD >3. Almost all aggregators contain aromatic systems, whilst there are a significant number of non-aromatic drugs. The shape analysis merely served to underline the lack of 3D structure in both aggregators and drugs.
Aggregation is only really a confounding issue when the aggregation occurs at a concentration comparable to the observed biological activity. If the measured activity in the screen is single figure nanomolar then it is unlikely to be due to aggregation, however it is important to note that the same compound may actually be an aggregator in the micromolar range and if it appears as a weak hit in another screen it may be acting as an aggregator.
A vortex script to flag potential aggregators is available here.
Whilst aggregation can simply impact the assay technology and they are flagged in screens against multiple targets there are occasions when it can be target specific. In a program looking for inhibition of a protein–protein interaction involving tumor necrosis factor α (TNFα) DOI it was shown that inhibition was the result of an ordered conglomerate of an aggregating small-molecule inhibitor (JNJ525).
The crystal structure showing the interaction is available (PDB 5MU8) and is displayed below using 3Dmol.js, the ligand (JNJ525) is shown in red..
Mouse Controls
| Movement | Mouse Input | Touch Input | ||
|---|---|---|---|---|
| Rotation | Primary Mouse Button | Single touch | ||
| Translation | Middle Mouse Button or Ctrl+Primary | Triple touch | ||
| Zoom | Scroll Wheel or Second Mouse Button or Shift+Primary | Pinch (double touch) | ||
| Slab | Ctrl+Second | Not Available |
Worth reading
Solubility at the Molecular Level: Development of a Critical Aggregation Concentration (CAC) Assay for Estimating Compound Monomer Solubility DOI
Last updated 29 March 2017