Cytochrome 2D6 inhibition
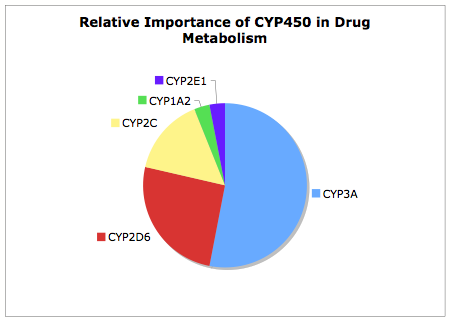
It is useful to remember of the 57 Human CYP Enzymes few have major role in drug metabolism, as can be seen from the chart below CYP2D6 is the second most important contributor to CYP450 mediated metabolism, this together with CYP3A accounts for over 75% of the CYP450 mediated metabolism.
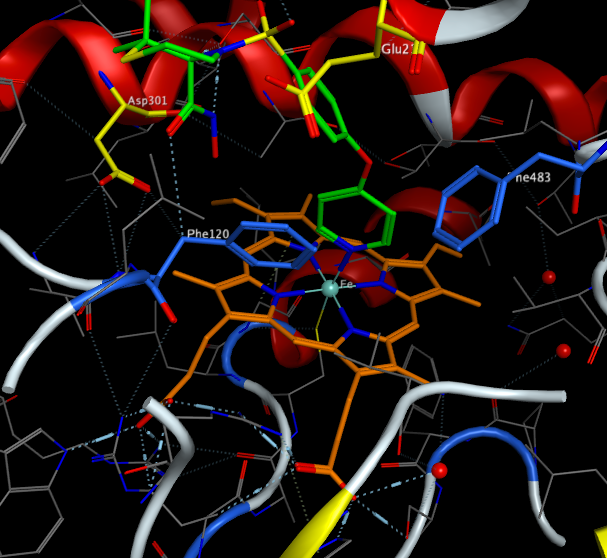
Active site of CYP2D6
A crystal structure of CYP2D6 has been published 3TDA showing the pyridyl ring of Prinomastat (highlighted in green) coordinating with the iron of the porphyrin ring. It also shows the two carboxylic acids (Asp301 and Glu216) highlighted in yellow that are thought to be responsible for the selectivity for basic ligands. Two phenylalaninies (Phe120 and Phe483) highlighted in blue offer the potential for hydrophobic or pi-stacking interactions.
CYP2D6 Inhibition Data from ChEMBL
ChEMBL is a database of bioactive drug-like small molecules, it contains 2-D structures, calculated properties (e.g. logP, Molecular Weight, Lipinski Parameters, etc.) and abstracted bioactivities (e.g. binding constants, pharmacology and ADMET data). Currently it contains data for 1,359,508 molecules and 9,414 different targets. ChEMBL contains data for CYP2D6 inhibitors, I downloaded all the IC50 data and imported it into Vortex, I then deleted those results that the ChEMBL team had flagged as potentially unreliable. Then I calculated a number of physicochemical properties using a variety of scripts.
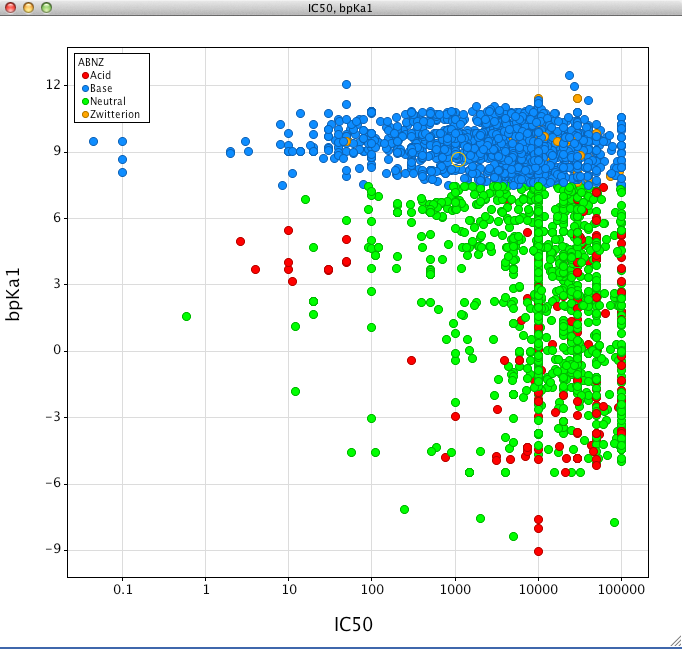
Looking at the scatter plot below (IC50 v calc LogP) it is noticeable that in general zwitterions do not inhibit CYP2D6 in addition few acids display high affinity except for a group of very lipophilic compounds. In contrast the plot would suggest basic compounds are more of a concern. The plot also suggests that lipophilicity may have an influence on activity.
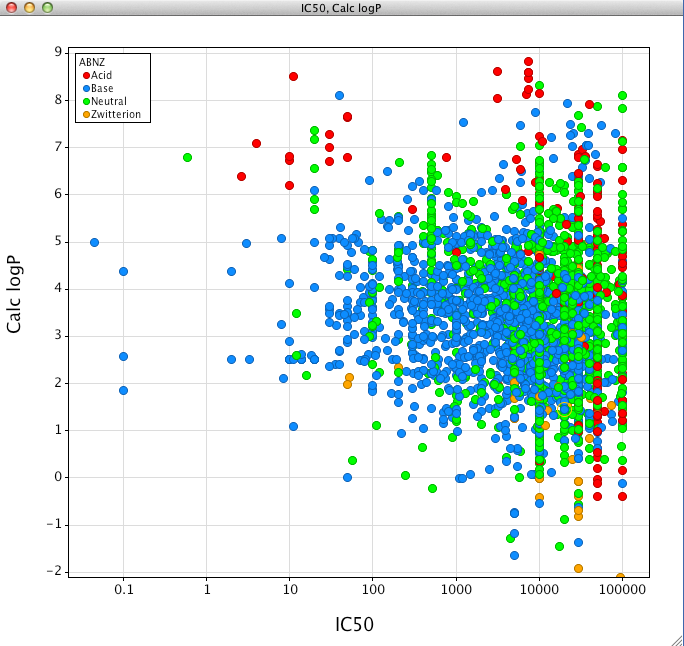
Indeed if we plot the calculated most basic pKa versus IC50 we can more clearly see that the majority of the more potent CYP2D6 inhibitors are basic. This would be consistent with the presence in the active site of the two key acidic residues Asp301, Glu216 and several aromatic residues, Phe483 and Phe120, that could all potentially act as key binding residues for basic inhibitors.
It has been reported DOI that Polar surface area has a significant influence on 2D6 inhibition, looking at the ChEMBL dataset it seems that the CYP2D6 affinity of acids and zwitterions are not influenced by polar surface area. However as can be seen in the plot below for neutral compounds and to a lesser extent basic compounds, those with higher polar surface area are less prone to 2D6 inhibition.
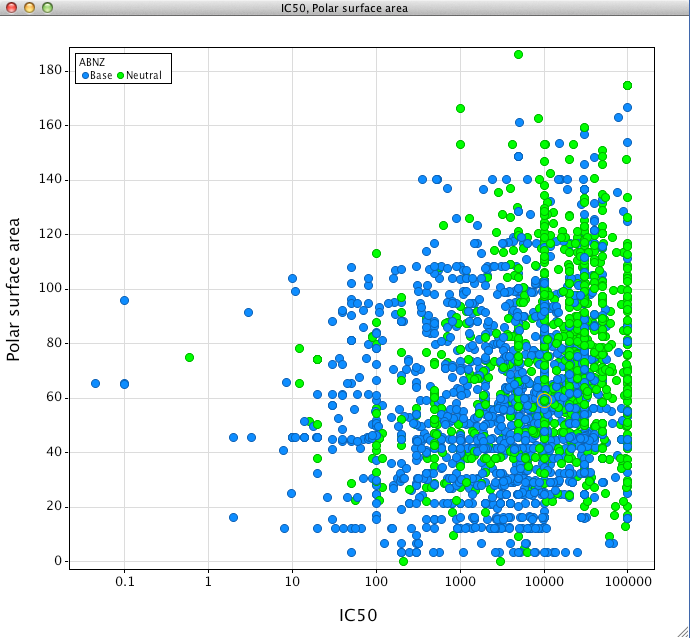
Looking at the neutral compounds first, of the compounds with a polar surface area of > 100 only 5 (2%) compounds had a CYP2D6 IC50 < 1uM. In contrast for compounds with a polar surface area of <50 around 46 (17%) had a CYP2D6 IC50 < 1uM.
For basic compounds, of the compounds with a polar surface area of > 100 only 30 (17%) compounds had a CYP2D6 IC50 < 1uM. In contrast for compounds with a polar surface area of <50 around 183 (26%) had a CYP2D6 IC50 < 1uM.
Matched Pair Analysis
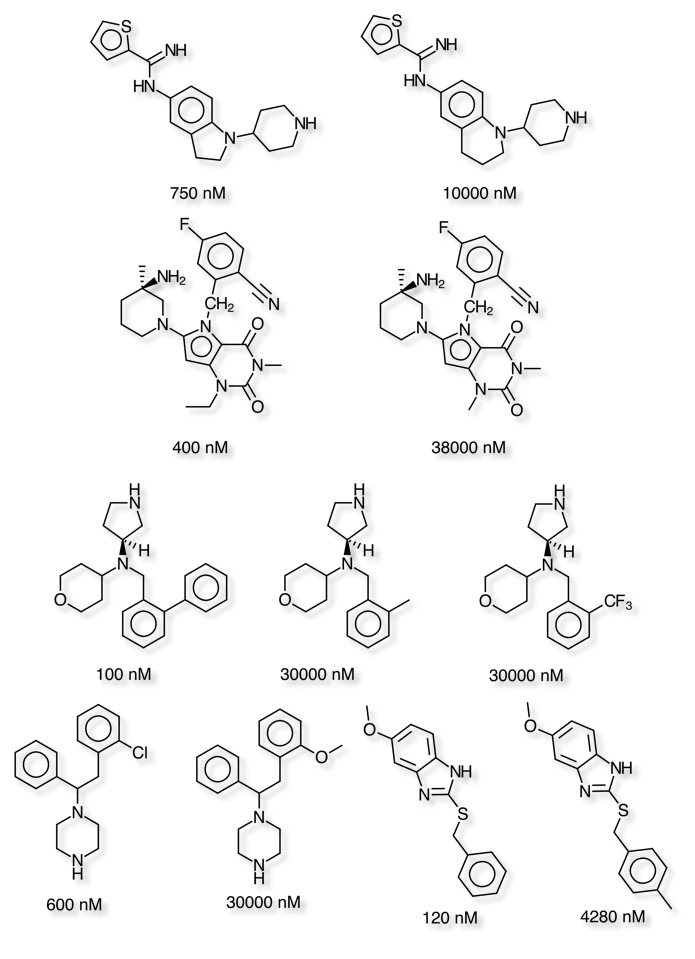
Using Vortex to conduct a matched pair analysis helps to identify small changes that have a significant influence on the CYP3A4 inhibition. As might be expected many of the more potent inhibitors have a heterocycle that can coordinate to the iron in the haem ring of the cytochrome. The matched pair analysis identifies a number of examples where modification of the heterocycle has a significant effect on inhibition. Either by sterically encumbering the nitrogen proposed to coordinate to the iron, or by reducing the electron density on the nitrogen. An alternative approach has been to replace a pyridine with the bioisosteric fluoro or chloro phenyl. These results mirror those described in more detail on the CYP3A4 inhibitors page.
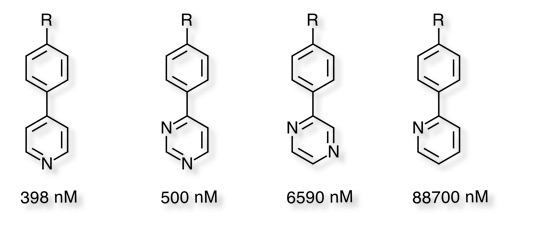

The matched pair analysis also shows how relatively small changes can have a significant effect on CYP2D6 inhibition, from ring expansion to simple aromatic substituent changes. Some of these changes would not be expected to have significant effects on the overall physicochemical properties of the molecule and possibly reflect unfavourable steric interactions. Many of these compounds are also less likely to be binding to the iron atom and are thus probably binding in a different pose.
See also
Last Updated 22 July 2014