Brain Penetration (a work in progress)
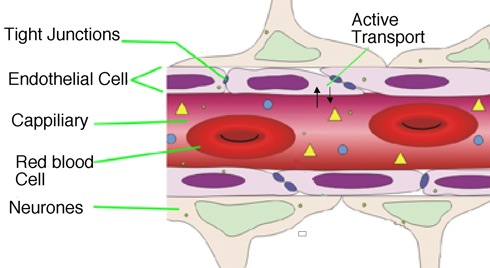
The brain is protected from xenobiotic agents by the blood-brain barrier (BBB). The BBB is a network of capilliaries lined by endothetial cells characterised by lack of fenestrations and very tight junctions between the cells. This restricts paracelleular diffusion of molecules, in addition there are a number of active transport mechanisms for transporting molecules into and more abundantly out of the brain. There are a number of energy dependent transporter proteins that are capable of binding to a wide variety of substrates and pumping them back into the plasma, perhaps the best known of which is multidrug resistance protein (MDR). In addition there are a number of specific carriers for essential molecules (amino acids, glucose, nucleosides) that transport ligands into the CNS.
In some diseases the blood-brain barrier may be disrupted, a number of pathogens can causes inflammation of the membranes that surrounding the brain and spinal cord (meninges), when the meninges are inflamed, the blood–brain barrier may be disrupted. Several auto-immune diseases such as Multiple Sclerosis and Devic’s disease are also thought to cause disruption of the blood brain barrier. Disruption of the blood-brain barrier has also been suggested in Stroke and Alzheimer’s Disease DOI. One potential beneficial effect might be that it allows improved drug access to the CNS.
Treatment of brain tumours can be of limited efficacy due to the impact of the blood-brain barrier impeding drug access, it has been shown that a hyperosmolar mannitol infusion can disrupt the blood-brain barrier DOI.
In vitro models of the BBB.
Several artificial membranes have been used to model BBB penetration, however most lack the correct morphology and expression levels of the transporter proteins, in particular the Caco-2 cell line used to predict intestinal absorption very poorly predicts brain penetration. The MDCK cell line transfected with the multi-drug resistance gene (MDR-MDCK) has been used to model BBB penetration and has been shown to correlate with in vivo studies (Int. J. Pharm. 2005, 288, 349). Whilst these models do give an estimate of the intrinsic ability of a molecule to cross the membrane you need to bear in mind that binding to plasma proteins can also limit brain penetration in vivo. In addition the intrinsic pharmacokinetics of the compound may overcome any liabilities identified in vitro.
In vivo studies
Probably the most often used measure of brain penetration is the partition between blood plasma and whole brain (log BB), whilst relatively straight forward to measure this can be highly misleading. Many highly lipophilic molecules partition extensively into the brain tissue but are not available to act on the target protein. It is much better to either measure the free unbound drug concentrations or use a receptor occupancy or pharmacological assay if possible. There has been increased use of imaging techniques to more accurately measure both BBB penetration and target protein occupancy, whilst enormously valuable the development of a PET tracer can require as much effort as a drug discovery program! Micro-dialysis and ex-vivo binding assays provide very high quality information but do require dedicated expertise.
In man the most simple way to study the entry of drugs into the CNS is to measure drug concentrations in the lumbar CSF collected by a single lumbar puncture following a bolus or continuous drug infusion. However the significant limitations of this method will no doubt encourage the use of noninvasive characterisation of drug entry into the CNS by magnetic resonance imaging (MRI) or positron emission tomography (PET).
Molecular Properties Effecting BBB penetration
As might be expect lipophilicity has a major effect on BBB penetration, and early studies highlighted the parabolic relationship between LogP and CNS activity in vivo in rodents. A recent study (Med. Chem.: Cent. Nerv. Syst. Agents 2002, 2, 229-240 ) looking at BBB penetration of CNS active drugs provides useful guidelines.
Suggested Physicochemical Property Ranges for Increasing the Potential for BBB Penetration.
| Property | top 25 CNS drugs mean | suggested limits | % of top 25 CNS drugs in suggested range | preferred range | % of top 25 CNS drugs in preferred range |
| PSA (A2) | 47 | <90 | 96 | <70 | 76 |
| HBD | 0.8 | <3 | 100 | 0-1 | 92 |
| cLogP | 2.8 | 2-5 | 68 | 2-4 | 52 |
| clogD (pH 7.4) | 2.1 | 2-5 | 61 | 2-4 | 61 |
| MW | 293 | <500 | 100 | <450 | 100 |
Transporter mediated efflux of drugs is major issue in drug discovery. The best understood of the transporters is ABCB1 also known as P-gp, MDR1 (mdr1 in rodents) this transporter is present in the blood-brain barrier, in the gut, on hepatocytes, and the kidney.
Computational Models of Brain Penetration
There have been many attempts to build computational models to predict brain penetration, these range from simple LogP correlations to sophisticated machine learning methods. An example would be the Bayesian neural network described by Winkler and Burden DOI using an 85 compound dataset and a range of calculated descriptors including including counts of hydrogen-bond acceptors and donors, rotatable bonds, log P, molecular weight, and polar surface area (PSA). However when applied to test compounds unrelated to the test set the prediction becomes unreliable. This nicely underlines the critical nature of the dataset used, the models either seem to rely on a small number of hand curated compounds which can only reflect a limited amount of chemical space. Or very large data sets that were generated based on the therapeutic indication, unfortunately not all molecules patented to treat Stroke/Alzheimer’s Disease etc. actually have good brain penetration and whilst other molecules may be intended to treat a peripheral target this does not mean they are not brain penetrant.
It is clear from many publications that a number of physicochemical properties influence central nervous system (CNS) penetration and it is often possible to play off one property against another in an effort to improve CNS penetration. An interesting paper from Wagner et al Moving beyond Rules: The Development of a Central Nervous System Multiparameter Optimization (CNS MPO) Approach To Enable Alignment of Druglike Properties DOI describes an algorithm to score compounds with respect to CNS penetration. The CNS MPO score was built based on six fundamental physicochemical properties: ClogP, ClogD, MW, TPSA, HBD, and pKa each weighted from 0 to 1.0 and most desirable and least desirable ranges for each physicochemical property is displayed in the table below.
| Property | Transformation (T0) | Weight | More desirable range (T0 = 1.0) | Less desirable range (T0 = 0.0) |
|---|---|---|---|---|
| ClogP | monotonic decreasing | 1.0 | ClogP <= 3 | ClogP > 5 |
| ClogD | monotonic decreasing | 1.0 | ClogD <= 2 | ClogD > 4 |
| MW | monotonic decreasing | 1.0 | MW <= 360 | MW> 500 |
| TPSA | hump function | 1.0 | 40 < TPSA <= 90 | TPSA <= 20; TPSA > 120 |
| HBD | monotonic decreasing | 1.0 | HBD <= 0.5 | HBD > 3.5 |
| pKa | monotonic decreasing | 1.0 | pKa <= 8 | pKa > 10 |
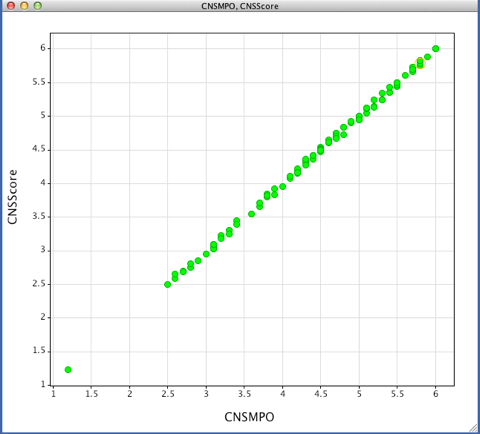
The calculated physicochemical properties used in the paper were obtained using a selection commercial packages: Biobyte for ClogP calculations, ACD/Laboratories for ClogD at pH 7.4, and ACD/Laboratories for pKa. TPSA was calculated using a sum of fragment-based contributions methodology described by in J. Med. Chem. 43, 3714–3717.. I recently wrote a script to implement this algorithm in Vortex. The plot shows a comparison of the score generated with the Vortex script (CNSScore) versus the values from the publication (CNSMPO).
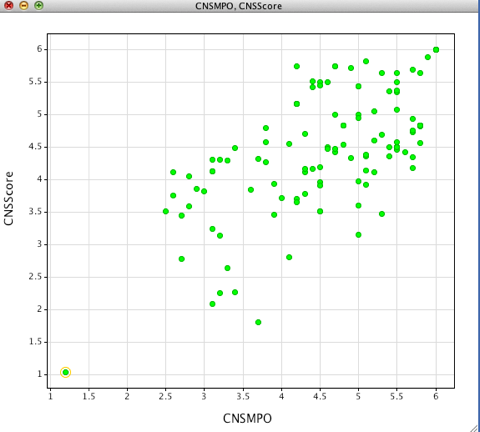
However whilst this recapitulated the results from the publication again when applied to molecules unrelated to the dataset I found it unreliable, in particular the dataset contains no compounds that are explicitly non-penetrant. A further complication was that when alternative tools were used to calculate the physicochemical properties perhaps unsurprisingly they failed to give the same results as shown below where ChemAxon tools were used to calculate properties.
That said one really nice feature of this implementation is that it is possible to see how each of the six different physicochemical properties contribute to the overall score and so I’ve decided to see if I can extend it’s utility.
The first stage is to compile a database of CNS penetrant or Non-penetrant molecules, to date I have data for around 800 compounds. This has required hand curation of literature reports and has been rather laborious. I’ve categorised compounds as penetrant or non-penetrant, any compounds for which the data is equivocal have been eliminated. I’ve also calculated a number of physicochemical properties.
Currently about 1/3 of the molecules are classified as non-penetrant and the PCA analysis shown below (penetrant=red, non-penetrant=green) suggests that the penetrant compounds cluster together whilst the non-penetrant are much more diverse.
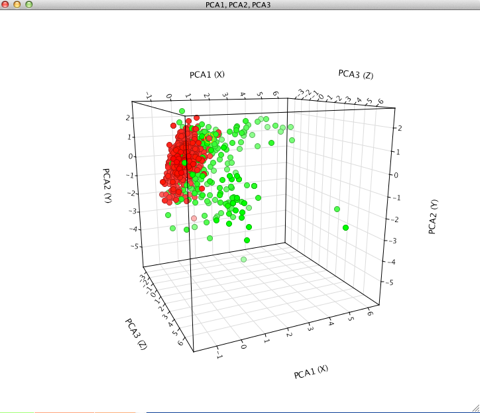
I’ve now written a modified Vortex script to calculate a CNSscore, the left hand plot is the actual CNS penetration assignment from the literature, the right the predicted score from the Vortex script.
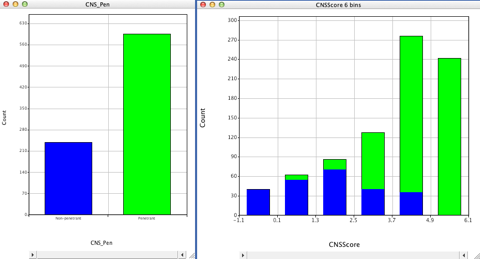
It is early days yet but showing promise.
A recent publication described "The Blood–Brain Barrier (BBB) Score" DOI a scoring function to determine the likelihood of a molecule being brain penetrant.
The blood–brain barrier (BBB) protects the brain from the toxic side effects of drugs and exogenous molecules. However, it is crucial that medications developed for neurological disorders cross into the brain in therapeutic concentrations. Understanding the BBB interaction with drug molecules based on physicochemical property space can guide effective and efficient drug design. An algorithm, designated “BBB Score”, composed of stepwise and polynomial piecewise functions, is herein proposed for predicting BBB penetration based on five physicochemical descriptors: number of aromatic rings, heavy atoms, MWHBN (a descriptor comprising molecular weight, hydrogen bond donor, and hydrogen bond acceptors), topological polar surface area, and pKa. On the basis of statistical analyses of our results, the BBB Score outperformed (AUC = 0.86) currently employed MPO approaches (MPO, AUC = 0.61; MPO_V2, AUC = 0.67). Initial evaluation of physicochemical property space using the BBB Score is a valuable addition to currently available drug design algorithms.
I'm often working on drugs targeting the CNS so it seemed a good idea to implement it as a Vortex script.
The data for over 1000 examples is provided in the supplementary information, this includes both CNS penetrant and non-penetrant compounds. The plot below compares the data from the supplementary information (SuppInf_BBBscore) with the data calculated for this implementation in the Vortex script. Whilst overall there is good agreement there appear to be a few outliers. On closer investigation many of the differences appear to be due to differences in the calculated TPSA. Since both implementations use the same ChemAxon software it is possible that updated version (I used version 19.8.0) has resulted in the differences.
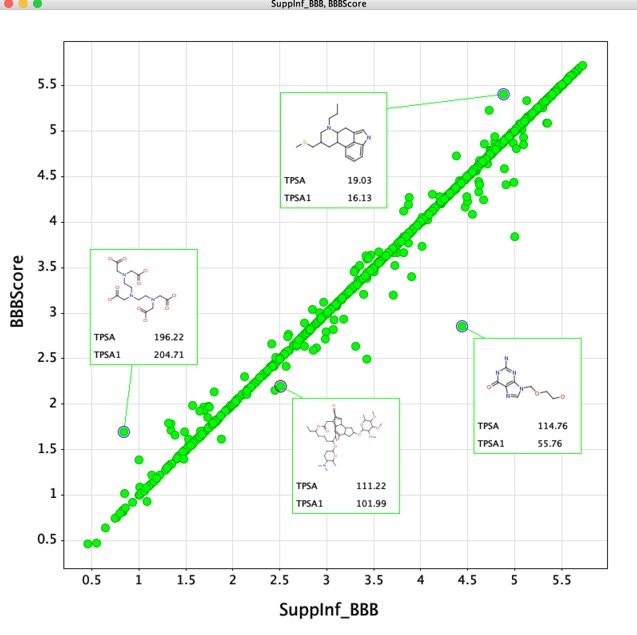
Details of the script and download are here
CNS Penetration of Kinase Inhibitors
A recent publication form Timothy Heffron, Small Molecule Kinase Inhibitors for the Treatment of Brain Cancer DOI discusses the issues with targeting brain and central nervous system cancers.
As will be discussed below, the majority of approved kinase inhibitors and kinase inhibitors that have advanced to clinical study have no report of CNS penetration, reportedly limited CNS penetration, or CNS penetration that is expected to be limited due to the action of the efflux transporters P-glycoprotein (P-gp) and breast cancer resistance protein (Bcrp).
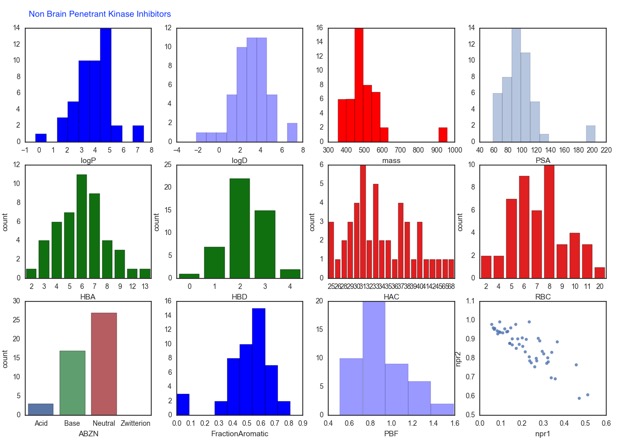
The supplementary information includes the names of the kinase inhibitors so I downloaded the structures from ChemSpider and used an iPython Notebook to calculate a range of physicochemical properties. The original paper compares median values of a number of properties but as the work described above makes clear there are a number of ways that brain penetration might be compromised and median values don't flag extremes of the range. As the plots below show in some ways the properties of both CNS-penetrant and nonCNS-penetrant are similar, however there are some features can be flagged. Among the nonCNS penetrant compounds there are some high molecular weight or polar surface area, there are also some extremes of Log P <0 and >7. The lack of acids in the CNS penetrant compounds is probably significant, with high plasma protein binding possibly being responsible. The non-CNS penetrant kinase inhibitors also have a greater number of hydrogen bond donors (HBD) this could also be exacerbated by the number of compounds predicted to be basic at physiological pH.
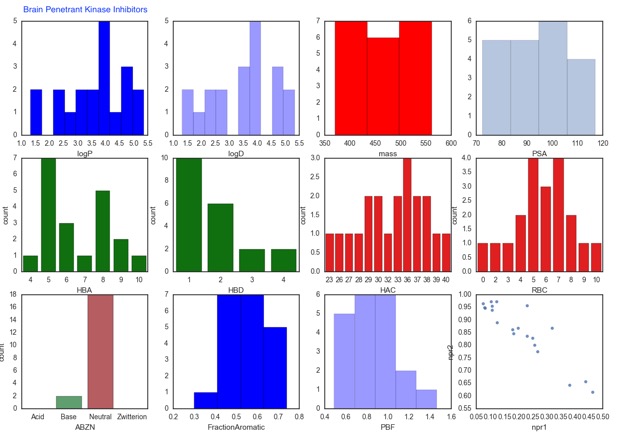
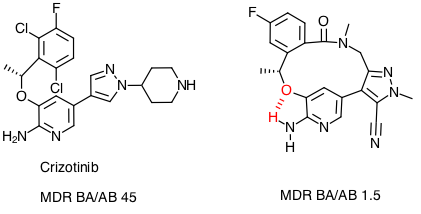
The paper also flags a number of potential strategies to improve CNS penetration in particular masking HBD via intramolecular hydrogen bonding, and macrocyclisation DOI.
Updated 6 December 2019