PROteolysis TArgeting Chimeras (PROTACs), Lysosome Targeting Chimeras (LYTACs)
Much of drug discovery deals with non-covalent, reversible interactions with the target protein, more recently there are also a class of small molecule therapeutic agents that bind covalently to the target protein these don't require the maintenance of high drug levels to maintain target occupancy. In contrast the PROteolysis Targeting Chimera (PRO- TAC) technology provides an alternative to module biological function by specially using the ubiquitin proteasome system to induce degradation of the target protein DOI.
PROTACs are bifunctional molecules that bind to the target protein and an E3 ligase, the simultaneous PROTAC binding of two proteins brings the target protein in close enough proximity for polyubiquitination by the E2 enzyme associated to the E3 ligase, which flags the target protein for degradation through the proteasome.
The PROTAC is composed of three components.
- A head-group that targets the protein of interest
- A crosslinker
- A second ligand at the opposite end that binds an E3 ligase
Ubiquitin-Proteasome Pathway (UPP)
PROTAC ligand binds to both the protein target and an E3 ubiquitin ligase to form a ternary complex, followed by transfer of ubiquitin from the E2 to the protein substrate. The ubiquitin is attached to a lysine on the target protein, subsequent ubiquitins are then added to a lysine residue of the first added ubiquitin. The ternary complex dissociates and PROTAC is recycled. Polyubiquitinated protein undergoes degradation via 26S proteasomes.
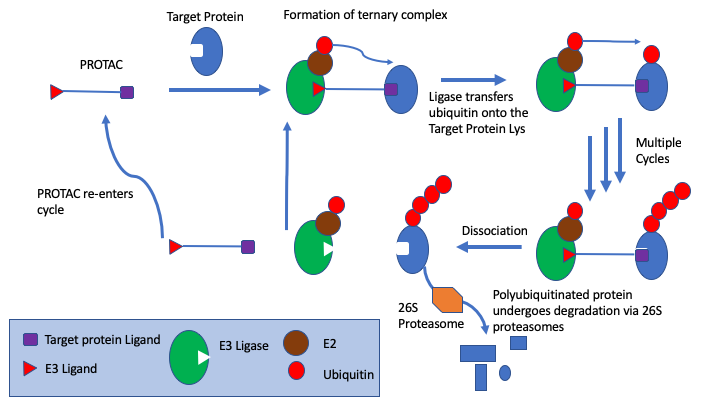
Since the efficacy depends on protein resynthesis rate, pharmacodynamic half-life is often much longer than the PK half-life similar to small molecules with very slow off-rates or covalent inhibitors. It should be noted that this is an intracellular mechanism and would probably unsuitable for some targets e.g. plasma targets.
Ligands targeting the E3 ligases
The ubiquitin system is a major coordinator of cellular physiology through regulation of both protein degradation and signalling pathways. A E3 ubiquitin ligase is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognises a protein substrate, and assists or directly catalyses the transfer of ubiquitin from the E2 to the protein substrate. The ubiquitin is attached to a lysine on the target protein, subsequent ubiquitins are then added to a lysine residue of the first added ubiquitin. Humans have an estimated 500-1000 E3 ligases but as of yet, only a few have been validated or exploited for PROTAC development, specifically VHL, CRBN, MDM2, and cIAP1.
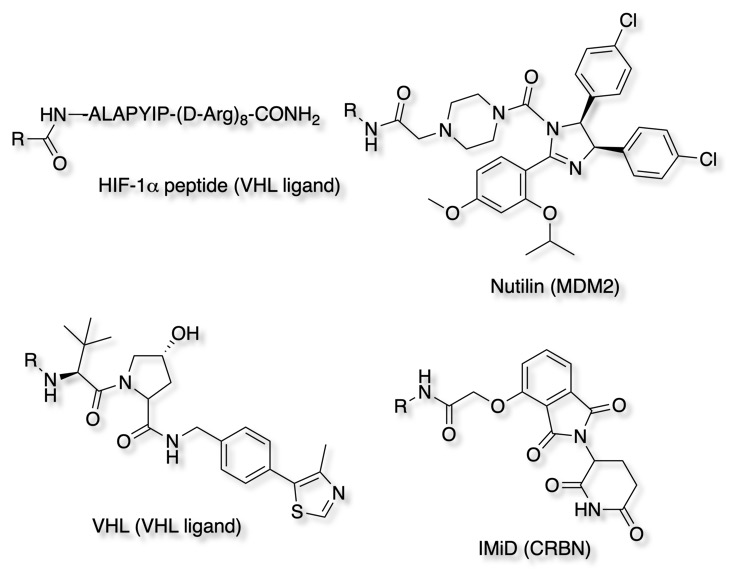
Early PROTACs used the HIF-1a peptide fragment bearing a cell-penetrating peptide sequence to recruit the Von Hippel-Lindau disease tumor-suppressor protein (VHL) E3 ligase. Later less peptide-like ligands were used still targeting the VHL E3 ligase, however these still suffered from poor cell penetration . The mouse double minute 2 homologue (MDM2) E3 was recruited by using a known MDM2-p53 PPI inhibitor, nutlin, as the E3 ligand with improved cell penetration. The E3 cereblon (CRBN) was identified as the molecular target of the immunomodulatory drugs, thalidomide, pomalidomide and lenalidomid.
Examples of Ligands for Target Proteins
Generally higher affinity ligands work better in Protacs but any ligand with Ki ~1uM or better has a good chance of working. Some work has used natural ligands/peptides but most of the recent work has focussed on the use of known small molecule inhibitors.
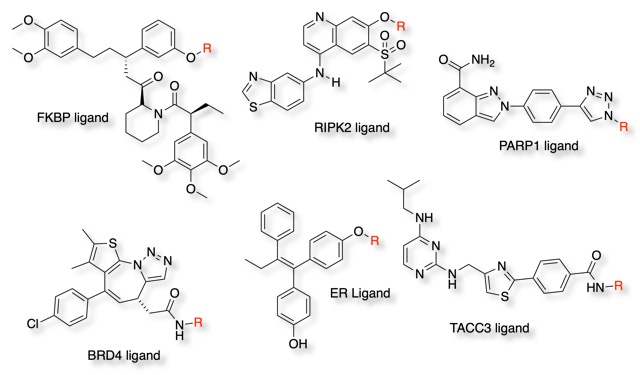
Many of the targeted proteins are oncology targets but other indications are also being investigated.
Protein Kinases (Akt, BCR‐Abl, c‐Abl, BTK, anaplastic lymphoma kinase [ALK], CDK9, EGFR, HER2, c-Met, TBK1, CK2, ERK1/2, FLT3, Fak, RIPK2, DAPK1, and PSD‐95)
Nuclear Receptors (ER, AR, and RAR)
Proteins in transcriptional regulation (BRD4, BDR9, Sirt2, HDAC6, TRIM24, IKZH1/3, Pirin, and Smad3)
Regulatory proteins (CRABP‐I/II, TACC3, AHR, FKBP12, ERRα, MCL1 and X‐protein)
Potential neurodegeneration targets (Huntingtin, Tau, a‐synuclein, and PSD‐95)
Metabolic enzymes (MetAP‐2 and DHODH)
DNA repair (PARP1)
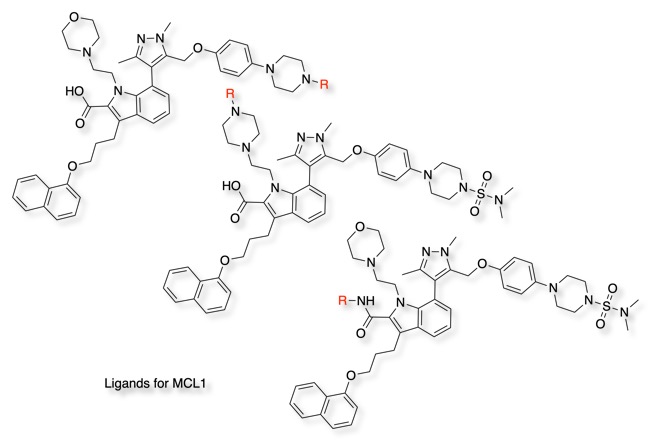
In order to identify the optimal vector for ligand attachment it may be necessary to explore a number of alternatives. In the work looking at designing PROTACS for MCL1, a critical pro-survival factor in cancers such as multiple myeloma where MCL1 levels directly correlate to disease progression. a variety of different attachment points on the MCL1 inhibitor A-1210477 were explored DOI.
Linker
The linker length and pole composition of PROTACs affect characteristics such as cell permeability, and solubility DOI these linkers contain a mixture of hydrophobic and hydrophilic functionality to balance the hydrophobicity/hydrophilicity of the resulting hybrid compounds.
Kinetics and Stoichiometry
One of the most attractive features of the approach is that a PROTAC molecule acts in a catalytic manner, it only needs to bind a molecule of target once to tag it for degradation, and then is released and set free to bind another molecule of target and carry on, as in a catalytic cycle DOI. Since the efficacy depends on protein resynthesis rate, pharmacodynamic half-life is often much longer than the PK half-life. The importance of the catalytic mechanism was underlined in a recent publication DOI, that compared both covalent binding and reversible binding PROTACs derived from the covalent BTK inhibitor ibrutinib. They demonstrated that a covalent binding PROTAC inhibited BTK degradation despite evidence of target engagement, while BTK degradation was observed with a reversible binding PROTAC.
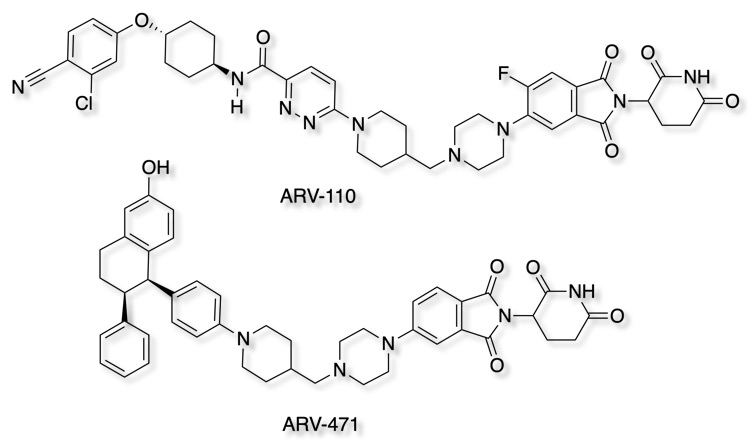
Whilst the physicochemical properties of these molecules are not ideal for oral administration, ARV-110 An oral androgen receptor PROTAC degrader for prostate cancer has been reported to be active after oral administration Poster.
Clinical Studies
The first PROTAC entered clinical trials in early 2019. ARV-110 targets the androgen receptor, a protein that contributes to the progression of prostate cancer. ARV-471 targets the estrogen receptor, which can cause certain breast cancer cells to grow unchecked.
The Phase 1 study will investigate the safety and tolerability of ARV-110 in patients with mCRPC who have progressed on at least two standard of care treatment regimens and includes exploratory measures of efficacy.
The structure of ARV-110 and ARV-471 are shown below.
Kymera have also described preclinical data on a targeted protein degrader of IRAK4 claiming pharmacokinetic and pharmacodynamic properties within tumours support advancement toward clinical development in MYD88-driven lymphomas in 2020. The table below summarises the current situation for both Protacs and molecular cues.
| Drug | Company | Target | Lead indication | Status |
|---|---|---|---|---|
| ARV-110 | Arvinas | Androgen receptor degrader | Prostate cancer | Phase II |
| ARV-471 | Arvinas | Oestrogen receptor degrader | Breast cancer | Phase II |
| ARV-766 | Arvinas | Androgen receptor degrader | Prostate cancer | Phase I |
| AR-LDD | Bristol Myers Squibb | Androgen receptor degrader | Prostate cancer | Phase I |
| DT2216 | Dialectic | BCL-XL degrader | Liquid and solid cancers | Phase I |
| KT-474 | Kymera/Sanofi | IRAK4 degrader | Autoimmune including AD, HS and RA | Phase I |
| KT-413 | Kymera | IRAK4 degrader with IMiD activity | MYD88-mutant DLBCL | Phase I |
| KT-333 | Kymera | STAT3 degrader | Liquid and solid tumours | Phase I |
| NX-2127 | Nurix | BTK degrader with IMiD activity | B cell malignancies | Phase I |
| NX-5948 | Nurix | BTK degrader | B cell malignancies and autoimmune | Phase I |
| CG001419 | Cullgen | TRK degrader | Cancer and other diseases | IND in 2021 |
| CFT8634 | C4 Therapeutics | BRD9 degrader | Synovial sarcoma | IND in 2021 |
| FHD-609 | Foghorn | BRD9 degrader | Synovial sarcoma | IND in 2021 |
| DKY709 | Novartis | Helios (IKZF2) degrader | Solid cancers | Phase I |
| CC-90009 | Bristol Myers Squibb | GSPT1 degrader | Acute myeloid leukaemia | Phase I |
| CC-92480 | Bristol Myers Squibb | Ikaros/Aiolos (IKZF1/3) degrader | Multiple myeloma | Phase I |
| CC-99282 | Bristol Myers Squibb | Ikaros/Aiolos (IKZF1/3) degrader | Lymphoma | Phase I |
| CFT7455 | C4 Therapeutics | Ikaros/Aiolos (IKZF1/3) degrader | Multiple myeloma and lymphoma | Phase I |
Lysosome Targeting Chimeras (LYTACs)
A recent publication highlights a new technology "Lysosome Targeting Chimeras (LYTACs) for the Degradation of Secreted and Membrane Proteins" DOI that further extends the protein degradation options beyond cytosolic proteins.
Targeted protein degradation is a powerful strategy to address the canonically undruggable proteome. However, current technologies are limited to targets with cytosolically-accessible and ligandable domains. Here, we designed and synthesized conjugates capable of binding both a cell surface lysosome targeting receptor and the extracellular domain of a target protein…. LYTACs represent a modular strategy for directing secreted and membrane proteins for degradation in the context of both basic research and therapy.
The PROTACtable genome
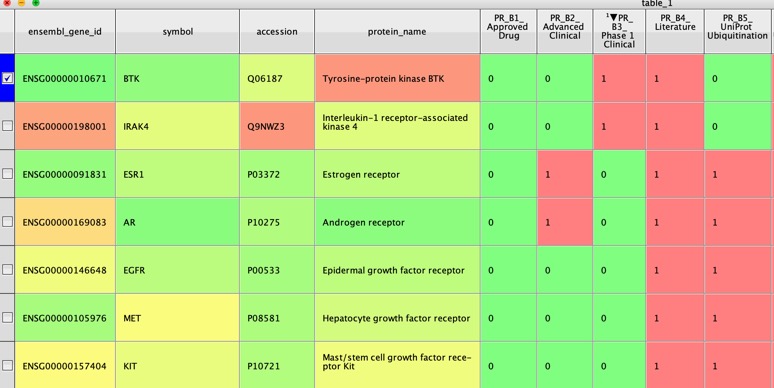
As Protacs have become more widespread the obvious question is which proteins are best suited to modulation by Protacs? A recent publication provides useful guidelines The PROTACtable genome DOI. The workflow is based on a method developed by a group at GSK, subsequently expanded and now integrated into the Open Targets Platform. Using publicly available data sources, the new method assesses whether a protein could be targeted using a PROTAC, based on the protein’s sequence, location, natural turnover rate in the cell, and evidence from published literature. The framework will help drug discovery researchers to gauge the PROTACtability of their protein of interest, and to prioritise their research accordingly.
Proteolysis-targeting chimeras (PROTACs) are an emerging drug modality that may offer new opportunities to circumvent some of the limitations associated with traditional small-molecule therapeutics. By analogy with the concept of the ‘druggable genome’, the question arises as to which potential drug targets might PROTAC-mediated protein degradation be most applicable. Here, we present a systematic approach to the assessment of the PROTAC tractability (PROTACtability) of protein targets using a series of criteria based on data and information from a diverse range of relevant publicly available resources. Our approach could support decision-making on whether or not a particular target may be amenable to modulation using a PROTAC. Using our approach, we identified 1,067 proteins of the human proteome that have not yet been described in the literature as PROTAC targets that offer potential opportunities for future PROTAC-based efforts.
Data for nearly 20,000 proteins are provided in the supplementary information.
Worth Reading
Protac-Induced Protein Degradation in Drug Discovery: Breaking the Rules or Just Making New Ones? DOI
PROteolysis TArgeting Chimeras (PROTACs) — Past, present and future DOI
PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer DOI.
First targeted protein degrader hits the clinic DOI
The PROTACtable genome https://doi.org/10.1038/s41573-021-00245-xDOI.
Last Updated 11 September 2021