Octopus trial for MS treatments
The Octopus trial is being led by researchers from the Queen Square MS Centre and the Medical Research Council (MRC) Clinical Trials Unit at University College London (UCL) and funded by the MS Society.
The multi-arm, multi-stage platform trial is designed to transform the way treatments for progressive MS are tested and will work up to three times faster than traditional trials.
More details…
https://www.ukri.org/news/participants-with-progressive-forms-of-ms-join-revolutionary-trial/.
More than 130,000 people live with MS in the UK and there are tens of thousands with progressive forms who have nothing to stop their MS getting worse. By tapping into the potential of approved drugs, which may have the potential to protect nerves, we can develop new treatments for MS faster.
COVID-19 Registered Trials
There are now a number of clinical trials underway and this review by The Centre for Evidence-Based Medicine provides an excellent summary of the trials that are taking place. They describe proposed pharmacological interventions and their mechanisms, when known, but unfortunately don't give the chemical structures.
Updated
I've also now included a few other structures that people have sent to me.
Here is the workflow I use to get the structures and access more information about the compounds.
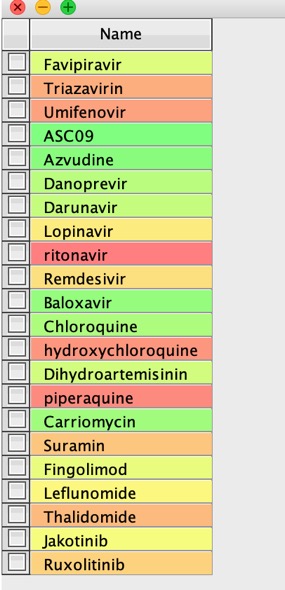
Create a text file with all the structures mentioned
ASC09
Azvudine
Azithromycin
Baloxavir
Carriomycin
Chloroquine
cobicistat
Danoprevir
Darunavir
Dihydroartemisinin
Favipiravir
Fingolimod
hydroxychloroquine
Jakotinib
Leflunomide
Lopinavir
marboxil
Methylprednisolone
oseltamivir
piperaquine
Remdesivir
ribavirin
ritonavir
Ruxolitinib
Suramin
Thalidomide
thymosin
Triazavirin
Umifenovir
Now read the text file into Vortex
The use a Name to Structure script to use a web service to get the structures, in this case I used ChemSpider. Now generate the InChiKey from the structures.
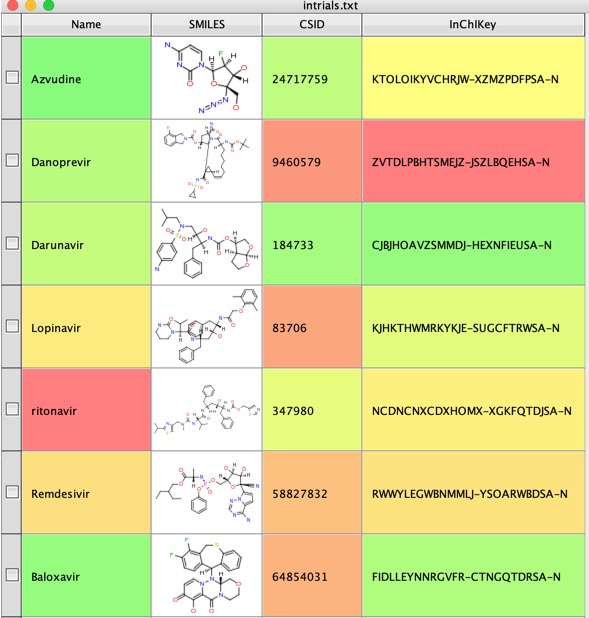
We can now use the InChiKey to search UniChem using another Vortex script to get identifiers for the molecule from various databases.
UniChem efficiently produces cross-references between chemical structure identifiers from different databases

We can then use the identifiers to search the various databases for more information
I've been asked if I could provide the structures for download
Here it is in SDF file format http://cambridgemedchemconsulting.com/news/files/COVID19/coviddata.sdf.zip
And in SMILES format http://cambridgemedchemconsulting.com/news/files/COVID19/forpost.smi.zip.
Who’s sharing their clinical trial results?
An interesting recent publication "Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study" DOI has highlighted the failure of many institutions to report the results of clinical trials within 1 year of completion as required by law.
4209 trials were due to report results; 1722 (40·9%; 95% CI 39·4–42·2) did so within the 1-year deadline. 2686 (63·8%; 62·4–65·3) trials had results submitted at any time. Compliance has not improved since July, 2018.
Thus nearly 60% of trials are not reported within the deadline, they also looked at the relative compliance of the different sectors
Industry sponsors and large (experienced) sponsors were most likely to report trial data, whereas universities were the least likely. The sponsor with the lowest compliance was the US government.
To aid monitoring they have produced FDAAA trials Tracker which allows anyone to check compliance.
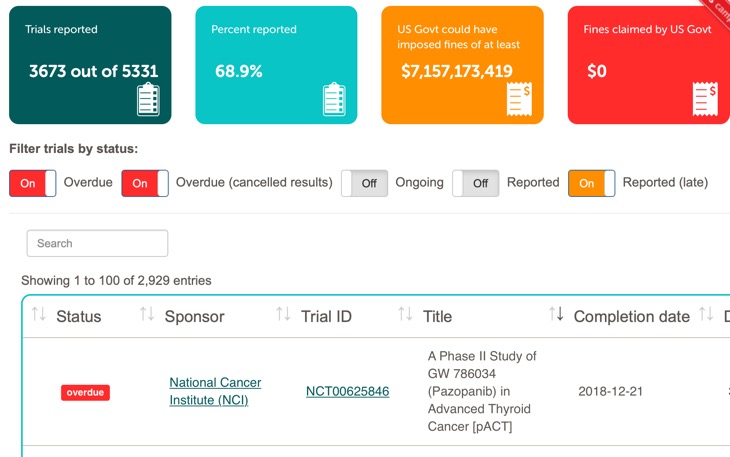
European universities dismal at reporting results of clinical trials
Clinical trial data is some of the most important information in Drug Discovery, after all it is humans we are looking to treat! However analysis of 30 leading institutions found that just 17% of study results had been posted online. The 30 universities surveyed are those that sponsor the most clinical trials in the EU. Fourteen of these institutions had failed to publish a single results summary.
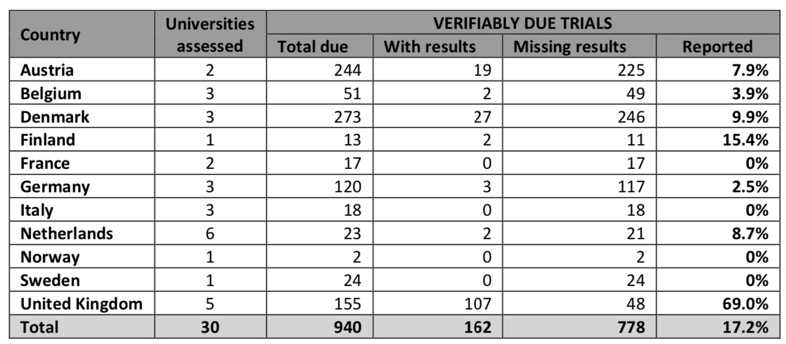
The Universities that have failed to publish a single trial result are highlighted in red in the table below.
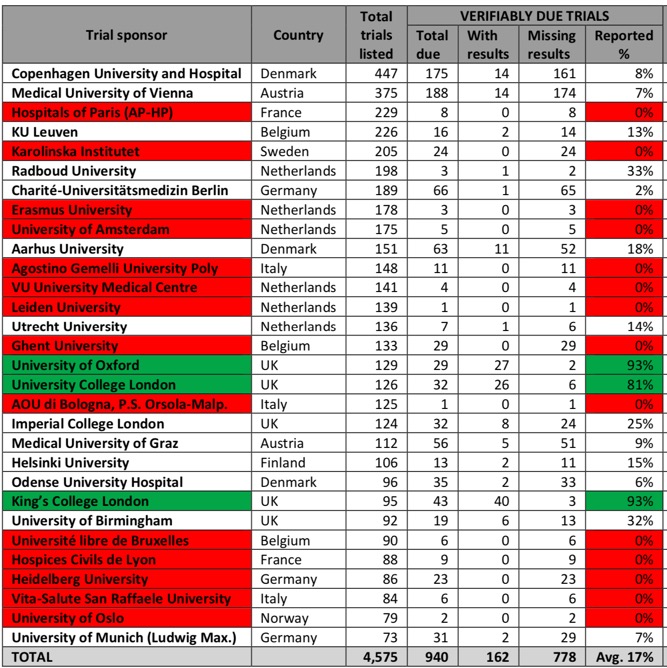
The contrast between the UK universities and the rest of Europe could not be starker,
UK universities in the survey performed significantly better than those in the rest of Europe. The University of Oxford and King’s College London had both published 93% of the trial results due on the register, and University College London had posted 81%.
According to the report every single medical university in the UK is currently working hard to upload missing clinical trial results onto the EU registry, and in many cases onto other registries such as ISRCTN and the US registry Clinicaltrials.gov as well. This demonstrates that where there is a will, there is a way.
If we remove the UK Universities from the analysis the level of reporting falls to a pitiful 7%.
Lack of transparency in clinical trials harms patients. The timely posting of summary results is an ethical and scientific obligation.
Real-Time Oncology Review Pilot Program
Clinical trials can be extremely lengthly and there have been many discussions about how to get medicines to patients more efficiently than the seemingly bureaucratic process that is currently in place.
Real-Time Oncology Review Pilot Program is a project to try and reduce the time needed to gain approval.
There are important caveats though.
Submissions to be considered for the RTOR pilot should meet the following criteria:
Drugs likely to demonstrate substantial improvements over available therapy, which may include drugs previously granted Breakthrough Therapy Designation for the same or other indications. Drugs meeting other criteria for other expedited programs (e.g. fast track, priority review) may also be considered. Straight forward study designs, as determined by the review division and the OCE. Studies conducted exclusively outside the United States and adjuvant, neoadjuvant, and prevention studies will be excluded. Endpoints that can be easily interpreted (for example, overall survival in a randomized trial). Supplements with CMC formulation changes and supplements with pharmacology/toxicology data will be excluded. Submissions with greater complexity, including those with companion diagnostics, may also be excluded for the purposes of the pilot program.
The real time review means the FDA can continuously review data as it is produced and give early feedback.
RTOR allows the FDA to review much of the data earlier, before the applicant formally submits the complete application. First, the applicant will present topline data for the FDA to determine whether RTOR would be appropriate for the supplement. If the agency determines RTOR is an appropriate review pathway, the applicant can start sending pre-submission data to the agency, under the original NDA/BLA, 2-4 weeks after all patient data has been entered and locked by the applicant in their database
This sort of process may be ideal for some indications where the trials give clear end points, survival in oncology, clearance of parasite in Malaria or other infectious diseases. Clinical trials for Psychiatric disease, marginal improvements over existing therapy or slowly progressing neurological diseases will probably not be suitable.
Why Most Clinical Research Is Not Useful
An interesting publication in PLOS Medicine titled “Why Most Clinical Research Is Not Useful” DOI.
John P. A. Ioannidis suggests that a series of features that make clinical research useful can be identified, including those relating to problem base, context placement, information gain, pragmatism, patient centeredness, value for money, feasibility, and transparency and concludes …
Overall, not only are (clinical) most research findings false, but, furthermore, most of the true findings are not useful. Medical interventions should and can result in huge human benefit. It makes no sense to perform clinical research without ensuring clinical utility. Reform and improvement are overdue.
Given the costs involved I suspect this final point may catch the eye.
Reform is needed. Altering our approach could easily produce more clinical research that is useful, at the same or even at a massively reduced cost
BIO Releases Clinical Development Success Rates 2006-2015
Biotechnology Innovation Organisation (BIO) have released the results of a huge study on clinical development success rates.
The study included 9,985 clinical trails and covered a wide number of therapeutic ares including Allergy, Autoimmune, Cardiovascular, Chronic High Prevalence Diseases, Endocrine, Gastroenterology, Hematology, Infectious Disease, Metabolic, Neurology, Oncology, Ophthalmology, Psychiatry, Rare Diseases, Respirator, and Urology.
Key findings from the study include:
- Clinical trial programs that used selection biomarkers saw an overall likelihood of approval (LOA) from Phase I of 25.9%, compared to 8.4% when no selection biomarkers were used.
- The overall LOA from Phase I for all developmental candidates was 9.6%, and 11.9% for all indications outside of Oncology.
- Of the 14 major disease areas studied, Hematology had the highest LOA from Phase I (26.1%) and Oncology had the lowest (5.1%).
- Oncology drugs were approved the fastest of all 14 disease areas.
- Rare disease programs had higher success rates at each phase of development vs. the overall dataset.
- Chronic diseases with high populations had lower LOA from Phase I vs. the overall dataset.
- Phase II clinical programs continue to experience the lowest success rate of the four development phases, with only 30.7% of developmental candidates advancing to Phase III
Re-evaluation of the traditional diet-heart hypothesis
A great detective story and also serves to underline the need for all clinical trial data to be published and stored in a publicly accessible format.
Ramsden, of the National Institutes of Health, unearthed raw data from a 40-year-old study, which challenges the dogma that eating vegetable fats instead of animal fats is good for the heart. The study, the largest gold-standard experiment testing that idea, found the opposite, Ramsden and his colleagues reported on Tuesday in BMJ.