Who’s sharing their clinical trial results?
An interesting recent publication "Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study" DOI has highlighted the failure of many institutions to report the results of clinical trials within 1 year of completion as required by law.
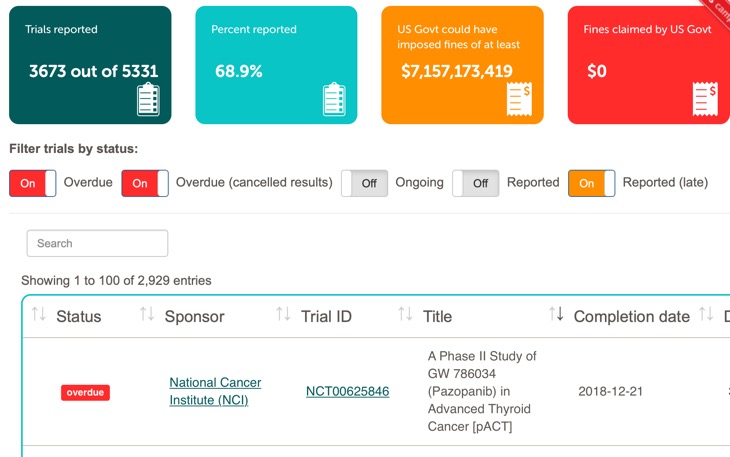
4209 trials were due to report results; 1722 (40·9%; 95% CI 39·4–42·2) did so within the 1-year deadline. 2686 (63·8%; 62·4–65·3) trials had results submitted at any time. Compliance has not improved since July, 2018.
Thus nearly 60% of trials are not reported within the deadline, they also looked at the relative compliance of the different sectors
Industry sponsors and large (experienced) sponsors were most likely to report trial data, whereas universities were the least likely. The sponsor with the lowest compliance was the US government.
To aid monitoring they have produced FDAAA trials Tracker which allows anyone to check compliance.