Find out more about the European Lead Factory
On Tuesday 30 November, members of the European Lead Factory (ELF) will participate in a webinar organised by the Young Scientists Network (YSN) of the European Federation of Medicinal Chemistry and Chemical Biology (EFMC).
The topic of the session is “Biological Testing of Hit Compounds”. Dr Vera Nies, Programme Manager at Lygature (the coordinating partner of the ELF) will give an introduction to the European Lead Factory. Her talk will be followed by a presentation entitled "Best practices in High Throughput Screening: ELF as an example", by Dr Steven van Helden of Pivot Park Screening Centre. The last ELF member presentation will be given by Dr Phil Jones of BioAscent, who will talk about the “Approaches Towards PAINSless Lead Generation”.
More details and registration here https://www.europeanleadfactory.eu/newsroom/elf-participate-efmc-ysn-webinar
ELF programs available for partnering
The European Lead Factory (ELF) is a collaborative public-private partnership aiming to deliver innovative drug discovery starting points. The ELFgives free access to up to 550,000 novel compounds, a unique industry-standard uHTS platform, and much more.
The success of the ELF approach has been widely acknowledged and the output has shown to be of high quality, worth following up and investment-ready. Funding for further development has been secured for several programmes, and by March 2018 two programmes have led to partnering deals being closed between the Programme Owner and an established pharmaceutical company.
A number of projects are now available for partnering.
The available projects are here.
European Lead Factory call for proposals
The closing date for submissions to the European Lead Factory is Friday 28th May 2021.
Following the UK’s exit from the European Union on 31 January, we are pleased to confirm that the European Lead Factory still welcomes screening proposals from UK researchers.
This is a fantastic opportunity for researchers to get access to a top class high-throughput screening platform.
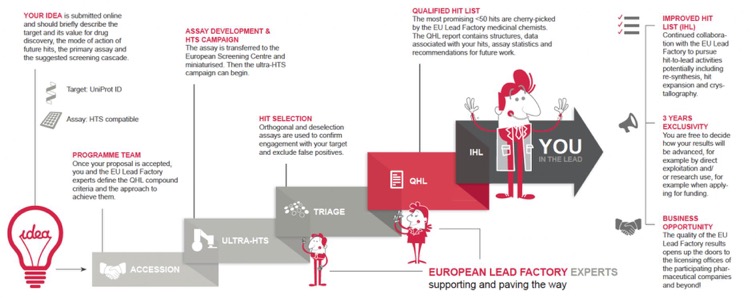
On this page, you can find the application forms, legal documents and all other relevant information required for your proposal submission.
The screening deck consists of around 535,000 compounds consisting of about 300,000 compounds from the original pharmaceutical companies, along with almost 190,000 compounds from custom design and synthesis. Furthermore, Grünenthal and Institut de Recherche Servier have provided around 50,000 new compounds not previously available to the European Lead Factory. Compounds from partners were selected for inclusion in the library based on several important quality criteria, as well as on the prerequisite that they are unique and not commercially available. Efforts were also taken to increase diversity, resulting in a top-quality compound library.
March webinar: High Content Screening at the European Lead Factory
The next European Lead Factory webinar will take place on Thursday 11 March from 11:00-12:00 (CET). In this webinar, we will focus on High Content Screening with a presentation by Professor Jason Swedlow, head of the National Phenotypic Screening Centre at the University of Dundee.
More details and registration here https://www.europeanleadfactory.eu/node/375
The single most important factor determining the likelihood of success of a project is the quality of the starting lead
Have you identified a disease mechanism that should be explored in a drug discovery project? Then you may have also considered developing a screening assay to find new chemical starting points that could serve as candidates for drug development.
In the next European Lead Factory webinar, taking place on Thursday 28 January, Dr Saman Honarnejad, Director of Drug Discovery at Pivot Park Screening Centre, will give a presentation on guidelines for HTS assay development, with an emphasis on methods to design, evaluate and improve conditions for biochemical and cellular assay formats.
The event will take place via Microsoft Teams on 28 January 2021 from 11:00-12:00 (CET). Discussions with the audience will form a key part of the webinar and time will be made available for questions and answers.
Registration is free using this link https://events.europeanleadfactory.eu/elf/elf-webinar/.
The European Lead Factory welcomes drug targets in all therapeutic areas. Interested? Submit your screening proposal now. The next deadline to apply will be in January 2021. Full details are on the ELF website.
I've been involved in a number of screening campaigns and I can confirm this is a high quality screening deck.
Still time to submit proposal to European Lead Factory
The closure date for the latest round of proposals for screening at the European Lead Factory is January 2021
The European Lead Factory welcomes drug targets in all therapeutic areas. Interested? Submit your screening proposal now. The next deadline to apply will be in January 2021.
Full details are on the ELF website.
I've been involved in a number of screening campaigns and I can confirm this is a high quality screening deck.
Proposals for European Lead Factory
The closure date for the latest round of proposals for screening at the European Lead Factory is January 2021
The European Lead Factory welcomes drug targets in all therapeutic areas. Interested? Submit your screening proposal now. The next deadline to apply will be in January 2021.
Also note
Following the UK’s exit from the European Union on 31 January, we are pleased to confirm that the European Lead Factory still welcomes screening proposals from UK researchers.
You can read more about the screening facility here https://www.europeanleadfactory.eu/node/353.
The details of how to submit are here https://www.europeanleadfactory.eu/how-submit/drug-target-assays/how-it-works.
I've written about the ELF here.

European Lead Factory Q&A
Interested in accessing a high quality high-throughput screening platform? Here is a chance to find out more about the European Lead Factory.
More details are here
The European Lead Factory (ELF) is a collaborative public-private partnership aiming to deliver novel lead molecules for drug discovery programs.
I've previously written about the ELF here.
Phenotypic Screening now offered by the European Lead Factory
The European Lead Factory has announced that it can now offer two types of phenotypic screening:
- A high-throughput, but “lower content” phenotypic approach that is suited to screening ELF’s entire compound collection, and
- A more complex “high content” screening approach using microscopy or flow cytometry to probe phenotype on a smaller subset of the compound collection
While low content assays can be live measurements or have fixed end points and involve well-averaged readouts, high content assays can be much more complex, based on live or fixed cells, multiple cell types and usually have more than one parameter as a readout. The complexity of the latter workflow makes it better suited to being performed on a smaller representative subset of the large collection.
Phenotypic screening historically has been the basis for the discovery of many drugs. Compounds are screened in cellular or animal disease models to identify compounds that cause a desirable change in phenotype. Only after the compounds have been discovered are efforts made to determine the biological targets of the compounds - a process known as target deconvolution.
Proposals for phenotypic screening approaches follow the normal review and selection process. A dedicated application form is available here.
The submission deadline for the next review and selection round is February 7, 2020.
European Lead Factory
Great news! European Lead Factory has restarted.
Pivot Park Screening Centre has successfully completed the first ultra-High Throughput Screening in IMI’s ESCulab project, and as such restarting the operations of the European Lead Factory. The screening on the European Compound Collection of ~500.000 compounds using a biochemical 1536-wells assay was finished within 4 days. Currently triaging of the UK owned program is ongoing within the Consortium, applying further biochemical and biophysical follow-up assays as well as the resynthesis of promising hits.
The programme is currently accepting proposals http://www.europeanleadfactory.eu/drug-target-assays.
European Lead Factory looking for novel screening programmes again
The European Lead Factory has been funded for another round of screening activities but under a new name European Screening Centre: unique library for attractive biology ESCulab. Over the next five years, the European Lead Factory will initiate 185 new drug discovery projects by screening medically relevant drug targets from European researchers, small and medium-sized enterprises and pharmaceutical industry against the ELF library of 550,000 unique chemical compounds.
The European Lead Factory was launched in 2013 and set up a joint collection of half a million compounds and a state-of-the-art high throughput screening centre. By the time the project ended last year, they had delivered results to researchers in universities, small biotechs and large companies across Europe, helping them to identify potential new drug candidates and breathing new life into a range of disease areas. In many cases, the seeds sown by the European Lead Factory resulted in new patents, partnering deals, and two start-ups. Now, a new IMI project, ESCulab will build on the work of the European Lead Factory. This means that researchers with drug targets can apply to screen the project’s compound collection for hits and get help developing any compounds further if they like. Jon de Vlieger, coordinator of the ESCulab consortium at Lygature, said: ‘It’s truly exciting to continue the onboarding of new and innovative proposals for screening and provide high quality starting points for drug discovery to academics and SMEs throughout Europe. In an effort to broaden our scope we are not only looking for target-based approaches, but now also enable phenotypic screens.’
You can apply here. If you don't have an assay in a format suitable for ultra high-throughput screening it is worth noting that the Wellcome Trust have small awards designed to help with the technology change required for HTS.
European Lead factory "Hit to Lead" workshop
The European Lead Factory hold annual meetings intended to support early career scientists, this years meeting will focus on "Hit to Lead optimisation". The meeting will be held at Janssen Pharmaceutica NV, Beerse, Belgium, November 6th - 7th 2017.
Full details are here https://www.europeanleadfactory.eu/early-career-researcher-event/.
Free participation (incl. reimbursement of travel costs up to € 250, accommodation, access to the social get-together) at the conference.
The draft agenda is here.
A great opportunity for those just starting on their drug discovery career.
The European Lead Factory Works!
The European Lead Factory (ELF) is a collaborative public-private partnership aiming to deliver novel lead molecules for drug discovery programs. When the consortium was formed around 5 years ago there was a lot of scepticism about whether a group of 30 partners rating from large Pharma companies to small academic groups could ever agree on a legal framework that would allow the ELF to function. In a addition, in an industry where confidentiality was critical to maintaining intellectual property the idea that a group of large Pharma companies would share their sample collections often regarded as the "Crown Jewels" seemed impossible. However I was at the European Lead factory Stakeholder Meeting (24-25 April 2017) and it is clear that is has been a success.
Another European Lead Factory success
We are now starting to see some of the results of the screening of academic projects at the European Lead factory that was initiated in 2013.
Dr Mahlapuu’s group, based at the University of Gothenburg, first identified a new target which could be used to reverse metabolic complications in type 2 diabetes. With the help of the European Lead Factory experts, she then screened the Joint European Compound Library of the then 320,000 industry compounds and identified a set of selective and potent small molecules which interfere with this target.
They have now formed a spinout company to develop these leads. ScandiCure has received 1 MSEK from the 2016 SWElife program to continue the development of first-in-class anti-diabetic drug based on small molecule antagonists of a novel key mediator - serine/threonine protein kinase 25 (STK25).
European Lead Factory Update
The latest newsletter from the European Lead Factory has just been published and highlights a number of notable achievements.
As a result of the screening campaigns >3000 qualified hits have been awarded to private and public target owners. 72 public target programmes have been accepted, 48 high throughput screens finished and 41 hit lists with associated data reports handed over to the target owners. >150 bespoke assays have been developed in order to extract the most interesting hits for public programmes.
There are now 450,000 compounds in the compound library of which 120,000 are novel compound specifically synthesised for the ELF.
You can read more details here https://www.europeanleadfactory.eu/results/
The European Lead Factory is a collaborative public-private partnership aiming to deliver innovative drug discovery starting points. Having established the first European Compound Library and the first European Screening Centre, the EU Lead Factory aims to give free access to up to 500,000 novel compounds, a unique industry-standard uHTS platform, and much more.
We are now starting to see publications describing these endeavours..
https://www.europeanleadfactory.eu/results/publications/scientific-articles/

