Drugs approved by EMA in 2019
I recently posted details of the small molecule drugs approved by the FDA in 2019. This generated considerable interest and I thought it might worthwhile doing a similar thing for the drug approvals in Europe. However this turns out to be less straight-forward, medicines can be authorised in several European countries simultaneously by using one of three procedures: the 'centralised procedure', the 'mutual-recognition procedure' or the 'decentralised procedure'. Medicines can also be authorised in a single Member State by using the national authorisation procedure of that country. The European Medicines Agency is responsible for the centralised procedure so I downloaded just the drugs approved via this mechanism.
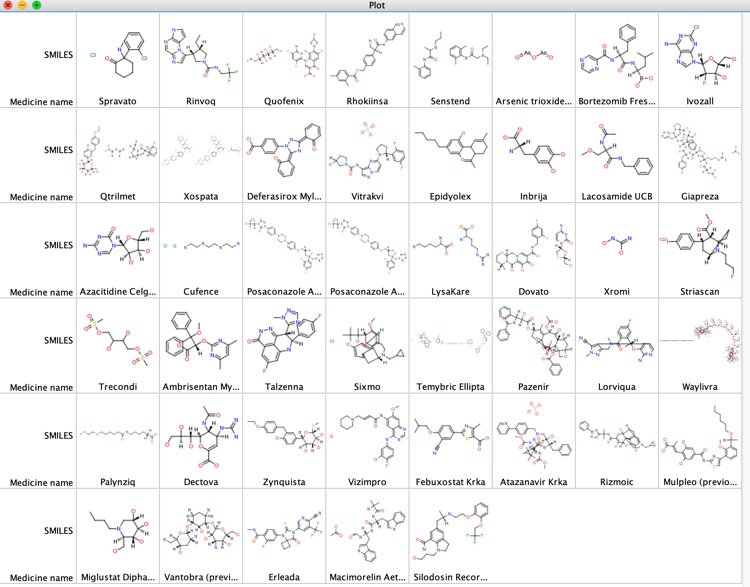
Of the 61 approvals in 2019, 45 were small molecule drugs and 16 were biologics. The structures of the small molecules are shown below
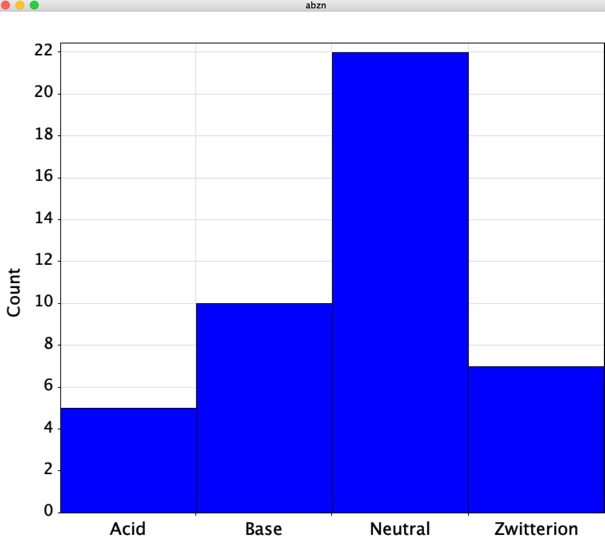
Looking at the calculated physiochemical properties of the small molecules one thing is quite interesting, around 50% are predicted to be ionised at physiological pH.
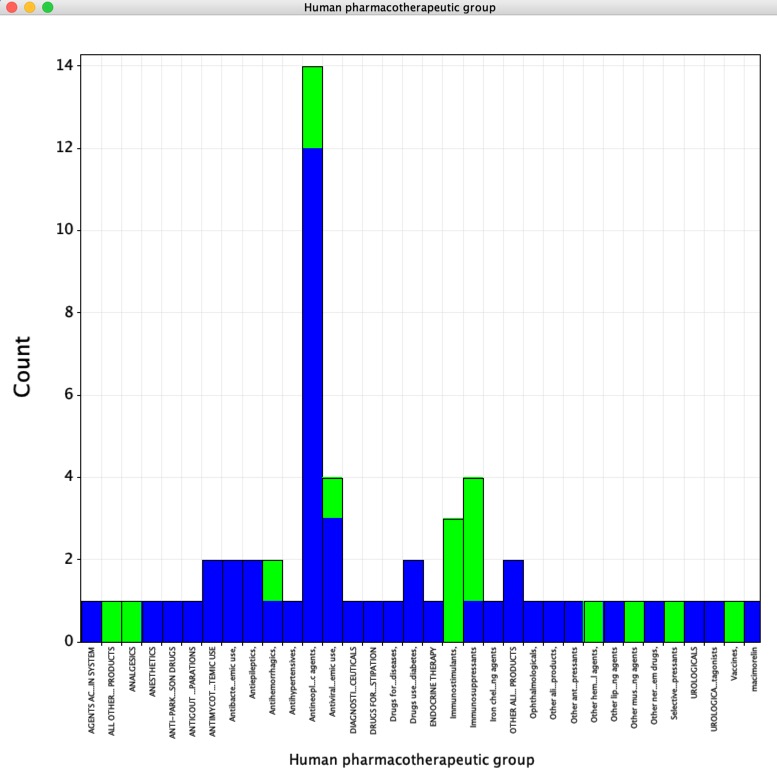
As shown in the plot below (Blue = small molecules, Green = Biologics) the largest group of drugs were Antineoplastic agents, the next largest groups being anti-virals and immunosuppressants.
Biosimilars
Four "biosimilars" were also approved. Kromeya and idacio both of which contain adalimumab (Humira) a TNF-alpha inhibitor as the active ingredient. Adalimumab was the first fully human monoclonal antibody approved by the FDA in 2002.
Grasustek contains the active substance pegfilgrastim (Neulasta) a PEGylated form of the recombinant human granulocyte colony-stimulating factor (GCSF) analog filgrastim. Zirabev contains the active substance bevacizumab (Avastin) that blocks angiogenesis by inhibiting vascular endothelial growth factor A (VEGF-A).
These four drugs join a number of other biosimilars approved in Europe, with the UK in particular keen to move to biosimilars. Biosimilars are expected to save the EU up to $44 billion in health care costs by 2020 LINK.
Most important, the EU is realizing the benefits of biosimilars without sacrificing safety or quality. Of the biosimilars approved since 2006, none have been withdrawn or suspended for safety or efficacy reasons. Further, regulators have not identified any differences in the nature, severity or frequency of adverse effects between biosimilars and biologics.