Dexamethasone shown to reduce COVID-19 mortality
The NIHR-funded and supported study RECOVERY (Randomised Evaluation of COVid-19 thERapY) has announced that the steroid dexamethasone has been identified as the first drug to improve survival rates in certain coronavirus patients.
A total of 2104 patients were randomised to dexamethasone once per day for ten days and were compared with 4321 patients randomised to usual care alone. Among the usual care control group, 28-day mortality was highest in those on ventilators (41%), intermediate in those on oxygen only (25%), and lowest among those who were not receiving any respiratory intervention (13%).
The study, conducted at the University of Oxford and led by Professor Peter Horby and Professor Martin Landray, found that dexamethasone reduced the risk of dying by one-third in ventilated patients and by one fifth in other patients receiving oxygen only. There was no benefit among those who did not need respiratory intervention.
Dexamethasone is an inexpensive corticosteroid medication used to treat many inflammatory and autoimmune conditions, such as rheumatoid arthritis and bronchospasm. Glucocorticoids are part of the feedback mechanism in the immune system, which modulates certain aspects of immune function. They bind to the glucocorticoid receptor, and the activated complex up-regulates the expression of anti-inflammatory proteins and represses the expression of proinflammatory proteins.
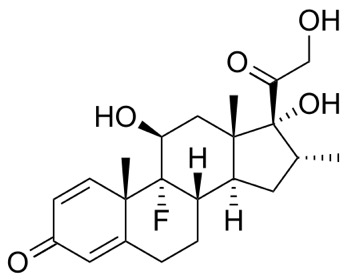
Dexamethasone CHEMBL384467 has good oral bioavailability (80-90%) and a reasonable half-life (4 h), with a high volume of distribution (> 50L). It is also available as a 3.3 mg/mL solution for intravenous use. Dexamethasone is extensively metabolised to 6-hydroxydexamethasone via CYP3A4 mediated oxidation.
The oral LD50 in female mice is reported to be 6.5g/kg.