Properties of clinical candidates
In an excellent publication "Where do recent small molecule clinical development candidates come from?" DOI, the authors give a detailed description on the development of clinical candidates from the initial hit. They also define the changes in physicochemical properties.
An analysis of physicochemical properties on the hit-to-clinical pairs shows an average increase in molecular weight (ΔMW = +85) but no change in lipophilicity (ΔclogP = −0.2), although exceptions are noted. The majority (>50%) of clinical candidates were found to be structurally very different from their starting point and were more complex.
I thought it might be interesting to look at the calculated properties of the 66 clinical candidates. Interestingly many have molecular weights > 500 and only around 30% contain an ionisable group. All structures contain an aromatic ring and 48 molecules contain 3 or more aromatic rings.
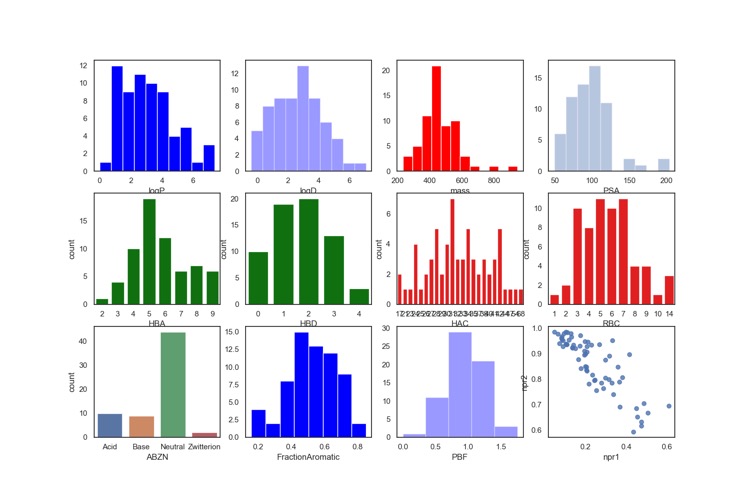
In case anyone was wondering the high molecular weight compounds are not peptides or macrocycles.
Molecular Interactions page updated
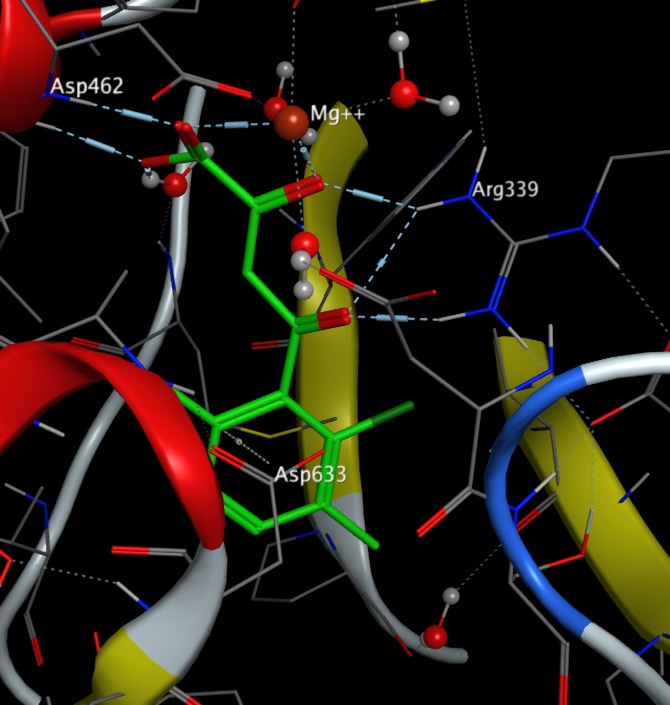
The Molecular Interactions page has been updated to include a section on anion-aryl interactions.
Drug Discovery Resources Updated
I've made a few additions and updated to the Drug Discovery Resources pages. In particular I've updated the covalent inhibitors page and added additional examples to the molecular interactions page. I've also started updating the ADME section and added a page on half-life and how it might be modulated.
Molecular Interactions page updated
I've updated the molecular interactions page to add more details on halogens.
Halogens are present in around 25% of drugs, calculated using data from Guide to Pharmacology Database and often used as bioisosteric replacements for H, Methyl, OH and NH2.
Bonds to halogen are significantly weaker than hydrogen bonds but there are many examples in the PDB of carbonyls interacting with halogens with bonds to I, and Br predominating. It is perhaps worth noting that halogens have been introduced into ligands to aid X-ray analysis, but they may also influence binding. Based on the observed bond angles the interaction is between the halogen and pi-cloud of the carbonyl rather than the lone pair with a clear clustering of X--O=C-N dihedral angles of 90° associated with interactions that involve primarily the pi-system of the carbonyl.
European Lead factory "Hit to Lead" workshop
The European Lead Factory hold annual meetings intended to support early career scientists, this years meeting will focus on "Hit to Lead optimisation". The meeting will be held at Janssen Pharmaceutica NV, Beerse, Belgium, November 6th - 7th 2017.
Full details are here https://www.europeanleadfactory.eu/early-career-researcher-event/.
Free participation (incl. reimbursement of travel costs up to € 250, accommodation, access to the social get-together) at the conference.
The draft agenda is here.
A great opportunity for those just starting on their drug discovery career.
The role of solvent in ligand binding
p>The role of water in ligand binding is often ignored and I thought it might be useful to add information to the drug discovery resources section. In particular :-
Expanding the page on molecular interactions
and adding a page on Solvation and Desolvation
If you have time to have a look, any comments or suggestions would be very welcome.
Separation of PK and PD
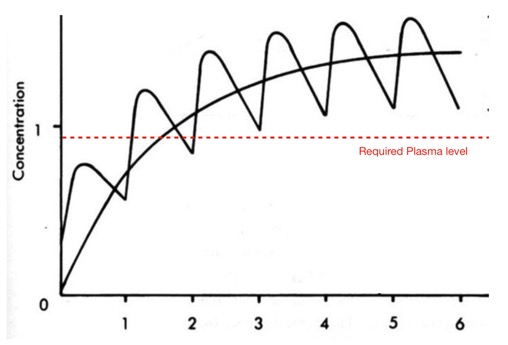
I’ve just added a section on compounds with extended off rates from the protein target.
For indications for which require an extended pharmacological profile a compound with a long binding half-life can have a duration of action which extends beyond the presence of drug levels in plasma needed for biological activity. In particular it may be possible to extend duration of action at the intended target whilst limiting activity at off-target proteins. Whilst this could be achieved by irreversible covalent binding there is now a growing body of evidence that many small molecules can display slow off rate kinetics